Globally, 284 million people are visually impaired, and 39 million people are blind, according to World Health Organization estimates.
If we add in those with near or distance vision difficulty – basically, anyone who wears prescription glasses — the number goes up to an estimated 2.2 billion people.
New technologies are helping us see better and giving hope to people with sight-threatening conditions affecting the retina or cornea of the eye. The market for assistive technology for people with vision impairment alone is projected to reach $7.6 billion within the next two years.
World Sight Day, October 12, presents a perfect opportunity to review groundbreaking Israeli solutions for visual health and wellbeing.
OrCam Technologies
OrCam was cofounded in 2010 by the same visionaries who founded Mobileye: Prof. Amnon Shashua and Ziv Aviram.
Shashua had an aunt who was gradually losing her sight to AMD. He decided to investigate how Mobileye’s AI-driven computer vision and machine learning technology, developed to support safe driving, could be adapted to help people with blindness or low vision to read.
The company now offers three reading aids:
- OrCam MyEye, a small box that clips onto eyeglasses and scans text or objects in front of it, and then dictates the content orally;
- OrCam Read, a handheld stylus that the user positions in front of the text and presses the button to hear the text dictated;
- OrCam Read 3, an all-in-one device to use as a customizable, next-generation magnifier for pictures, handwriting, math formulas, and text, as a handheld reading companion, or as a stationary reader.
OrCam products are used in 25 languages in 50 countries by people aged six to 100. An online Low Vision Help Center offers tutorials and troubleshooting.
CorNeat Vision
Founded less than eight years ago, CorNeat Vision pioneered a non-degradable tissue-integrating material technology, CorNeat EverMatrix, which biomechanically incorporates with eye tissue to provide safe, aesthetic and long-lasting remedies for corneally blind people and those suffering from glaucoma.
This material improves outcomes by minimizing the body’s natural immune system response to foreign bodies.
CorNeat’s EverPatch synthetic tissue substitute received FDA clearance in June for use in ophthalmic surgeries. The CorNeat KPro synthetic cornea is in clinical trials and is expected to receive initial marketing approval in 2024.
The company’s growing product portfolio also includes the CorNeat eShunt glaucoma drainage device and a periodontal regeneration membrane, the CorNeat gPatch.
Click here for the heartwarming story of Jamal Furani of Haifa, the first patient to receive the KPro synthetic cornea, in 2021.
Notal Vision
This Israeli-rooted digital healthcare startup – now headquartered in Virginia with R&D in Tel Aviv — develops remote monitoring services that include home devices for vision testing.
With FDA clearance since 2009, Notal now provides its ForeseeHome monitoring system to about 8,000 Americans with intermediate “dry” age-related macular degeneration (AMD) at high risk of developing the more advanced “wet” form of AMD, a disease of the retina.
AI algorithms analyze the data that patients generate at home, alerting the prescribing optometrist, ophthalmologist or retina specialist of any changes warranting their attention.
Notal Vision’s monitoring center, staffed by two ophthalmologists and 20 certified healthcare professionals, handles billing to insurance companies including the federal Medicare program for seniors.
The company is now developing a new service for patients in active therapy for wet AMD.
“Long-acting treatments today are administered only every few weeks to months, and it’s helpful for physicians to have remote access to what’s happening in the patient’s eye over time,” says CEO Kester Nahen, noting that the digital literacy of the elderly has grown tremendously since the pandemic and they often prefer to be monitored remotely.
The new product, Home OCT (Optical Coherence Tomography) uses a super compact version of the same imaging that retina specialists are familiar with, cheap enough to loan patients for use between office visits.
This platform, now under FDA review, is being used in clinical trials by pharma companies testing new AMD treatments. The company has raised over $100 million and is seeking an additional $20 million toward getting Home OCT in the market.
Samsara Vision
The lead product of Samsara Vision (previously VisionCare) is the Implantable Miniature Telescope (IMT), the first and only FDA-approved implantable medical device demonstrated to improve vision and quality of life in individuals with advanced, irreversible AMD, the leading cause of blindness in older Americans.
Advanced AMD causes blindness in the center of the field of vision, leaving peripheral vision intact.
Based on the principles of 17th century Italian physicist Galileo Galilei, the IMT combines unique biconvex and biconcave micro-lenses with air lenses to project a magnified image onto healthy retinal tissue surrounding the degenerated macula.
The magnification helps the healthy peripheral parts of the retina “see” the image viewed in straight-ahead vision despite the blind spot.
The IMT, developed 20 years ago by Isaac Lipshitz, has regulatory approval in the United States and Europe for placement in the eye by an ophthalmic surgeon.
Samsara Vision’s new-generation injectable SING IMT received a CE mark for the European Union in 2020 and is awaiting FDA approval. This updated device can be folded and inserted through a device delivery system that allows for a smaller incision and less complicated surgery.
AEYE
Primary-care physicians usually send patients with diabetes to an eye specialist for a yearly exam to check for a common vision complication, diabetic retinopathy.
AEYE’s FDA cleared desktop system allows the primary-care physician to perform this exam in less than two minutes without eye dilation.
Founded in late 2018, AEYE started selling its system across the United States at the beginning of this year.
Unlike many other AI health technologies, AEYE is not a decision-support platform for doctors but rather a diagnostic tool, points out Amit Wohl, VP Product & Marketing. This enables patients to be diagnosed conveniently without scheduling an appointment with a specialist.
The company is now raising funds and working toward getting clearance for a handheld version that can be used for diagnosing bedridden people.
“We also are working on study protocols to get clearance for diagnosing two other retinal diseases — glaucoma and AMD,” says Wohl.
EyeYon Medical
EyeYon has been developing cornea-focused sight-saving solutions for more than a decade. Its two main products are EndoArt and HyperCL. Earlier this year, EyeYon raised a $26 million Series C round.
EndoArt is the first synthetic implant to cure corneal edema (fluid buildup), a condition causing poor vision and severe pain.
Rather than replace the cornea, EndoArt is attached to the back of the cornea in a minimally invasive procedure, preventing the transfer and buildup of fluids. The implant is approved for use in Europe and Israel so far. EndoArt is the only synthetic cornea product to receive Breakthrough Device designation from the FDA.
Typically, less than 5% of topical eyedrops reach the target area of treatment. HyperCL is a unique therapeutic soft contact lens designed to increase the contact time of topical drops on the cornea, improving treatment outcome.
CE-approved for continuous wear and in the commercialization stage across Europe and Asia, Hyper-CL is used to deliver medication for various corneal and post-surgical conditions and is clinically proven to relieve corneal abrasion, inflammation or infection.
NovaSight
NovaSight makes two eye-tracking-based solutions for accurate screening and treatment of early vision disorders.
The EyeSwift Pro vision assessment system, which has CE clearance, screens for multiple vision impairments within seconds, supporting ophthalmologists, optometrists, orthoptists and pediatricians.
CureSight, which is CE and FDA cleared, treats lazy eye (amblyopia) while children watch a video of their choice at home, replacing the outdated patching treatment.
NovaSight is also working to address the unmet need for myopia prevention and vision health monitoring with TrackSight, a software as a service (SaaS) solution utilizing computer monitors’ embedded webcams to perform eye-tracking. This solution, still development, is designed to run on laptops and tablets used for educational, professional, and leisure purposes.
EyeCan
EyeCan is developing a smart headband that aids blind and visually impaired swimmers stay in their lane and avoid obstacles. In the United States alone, some eight million visually impaired people swim at least three times a week.
Tomer Etinger and Amit Fisher founded EyeCan in 2022, with support from the Israel Paralympic Committee, based on their industrial design project as students at the Holon Institute of Technology three years previously.
Working independently of the cloud, the headband’s camera continuously scans the pool and sends vibration and sound data to the headband’s image processor.
The processor sends alerts to the swimmer via bone conduction – not through earphones, explains Fisher, because “hearing is the strongest sense of visually impaired people and we wanted to leave the ear open.”
Fisher says that in training, blind swimmers “want less sound and more vibration” whereas in competition they depend on sound to know when they’re approaching the end of the pool. Therefore, the device offers both.
EyeCan is now at the proof-of-concept stage and is being tested in case studies with 50 visually impaired swimmers in Israel. The company is raising funds to continue development.
DeepOptics
There are 1.8 billion people worldwide with presbyopia (reduction in near vision, such as for reading). The two common options are progressive (multifocal) lenses and bifocals, which each have limitations.
DeepOptics is developing personalized, connected glasses that dynamically correct vision impairment using pixelated liquid crystal (LC) lenses. The battery-operated lenses perform like the human eye, allowing wearers to see what they want, when they want, at far or close distances.
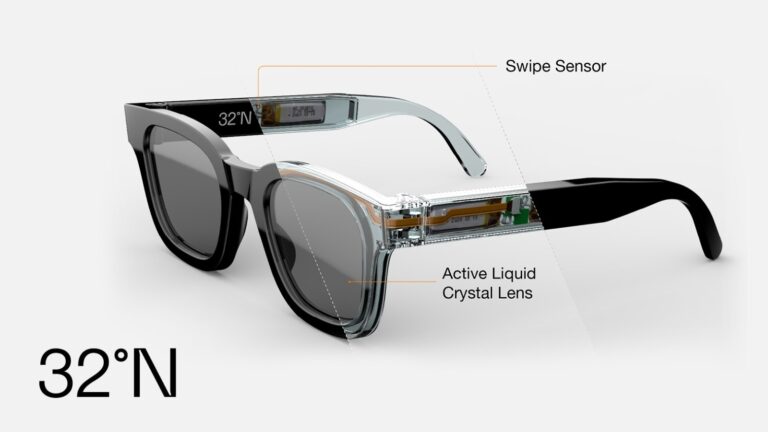
When the wearer swipes, a tiny processor embedded in the temple calculates the user’s personal prescription and commands millions of tiny pixels inside the lens to change their electrical state to bring close objects into focus.
The company’s debut product, 32°N adaptive sunglasses, was named a “Best Invention of 2022” by TIME magazine and by the CES consumer electronics show and is long-listed by Dezeen Awards 2023 in the Product Design (Consumer Design and Wearables) category.
TopoSpeech
The TopoSpeech sensory substitution device, being developed at Prof. Amir Amedi’s Ivcher Institute for Brain, Cognition & Technology at Reichman University, gives spatial information about the external world by applying sensory substitution with symbolic representations that correspond to the way our brains acquire and process information.
The system presents an auditory cue that enables the listener to locate an object’s exact location, including direction and distance from the listener.
TopoSpeech was presented by its inventors at the 2023 Israel Vision Science Society Conference at Bar-Ilan University’s School of Optometry and Vision Sciences. It is meant to be used with everyday headphones and earbuds.
Orasis Pharmaceuticals
Orasis is developing corrective eye drops for treating presbyopia, the loss of ability to focus on near objects as a result of the natural aging process.
Last February, the FDA accepted the company’s New Drug Application for review, based on results of two clinical trials, involving more than 600 patients, which evaluated the efficacy and safety of the product. Both trials met their primary and key secondary endpoints on Day 8, achieving statistically significant 3-line or more gain in distance-corrected near visual acuity and no loss of 1-line or more in distance visual acuity.
“We are encouraged by the acceptance of our NDA filing as we progress towards our mission of reshaping vision possibilities for the millions of people in the U.S. living with presbyopia, or blurry near-vision,” said Elad Kedar, Chief Executive Officer of Orasis Pharmaceuticals. “We look forward to working with the FDA towards approval and commercial launch of CSF-1.”