Age-related macular degeneration (AMD) is a leading cause of blindness in people over age 60. About 90 percent of those with AMD have the “dry” form for which there is no approved therapy.
And so the race is on to find a cure. The potential is huge, as products for treating the much smaller population of those with wet AMD ring up about $5 billion in annual sales.
The Israeli company Cell Cure Neurosciences in Jerusalem has thrown its hat in the ring with a treatment of injectable human retinal pigment epithelial (RPE) cells — the essential “helpers” for the eye’s photoreceptor cells — produced from pluripotent stem cells using a propriety technology.
CEO Charles Irving explains that with age, RPEs get run down and fail to provide the photoreceptors with the nutrients and pigments they need to function.
“The photoreceptor cells can only make it a little longer on their own before dying, and that’s irreversible,” he tells ISRAEL21c. “Our goal is to enable, for the first time, transplantation with new RPE cells so we can save the photoreceptors that haven’t already died and stop the progression of the disease.”
He likens AMD to a forest fire burning through the retina. The damage can be tolerated until it reaches the “houses in the village,” in this case the macula’s pinhead-sized fovea.
“If disease reaches the fovea, you have essentially lost central and color vision. We want to stop the ‘fire’ before it reaches the fovea. We do that by putting in young cells not susceptible to the aging process,” Irving says.
Clinical trials
Cell Cure Neurosciences’ OpRegen is being clinically tested for safety on advanced AMD patients at Hadassah University Medical Center in Jerusalem. The company won fast-track approval from the US Food and Drug Administration (FDA) for further trials in the United States.
The study is in four cohorts encompassing 15 patients: the first three patients received a low dose of OpRegen, the next three will receive a medium dose, and the following three will get the high dose intended for treatment. In the fourth cohort, also to be recruited in the United States early next year, six patients with less severe dry AMD will receive the high dose to test OpRegen’s efficacy at an earlier stage.
“These first studies are to show there is no harm in putting the new cells into the retina and that they will engraft properly and organize themselves to support the photoreceptor cells,” Irving says. “The next studies will look at whether they do their job to stop the progression of the disease.”
The ability of the cells to organize themselves – proven in the company’s animal studies — is critical to understanding the uniqueness of Cell Cure’s approach to regenerative medicine.
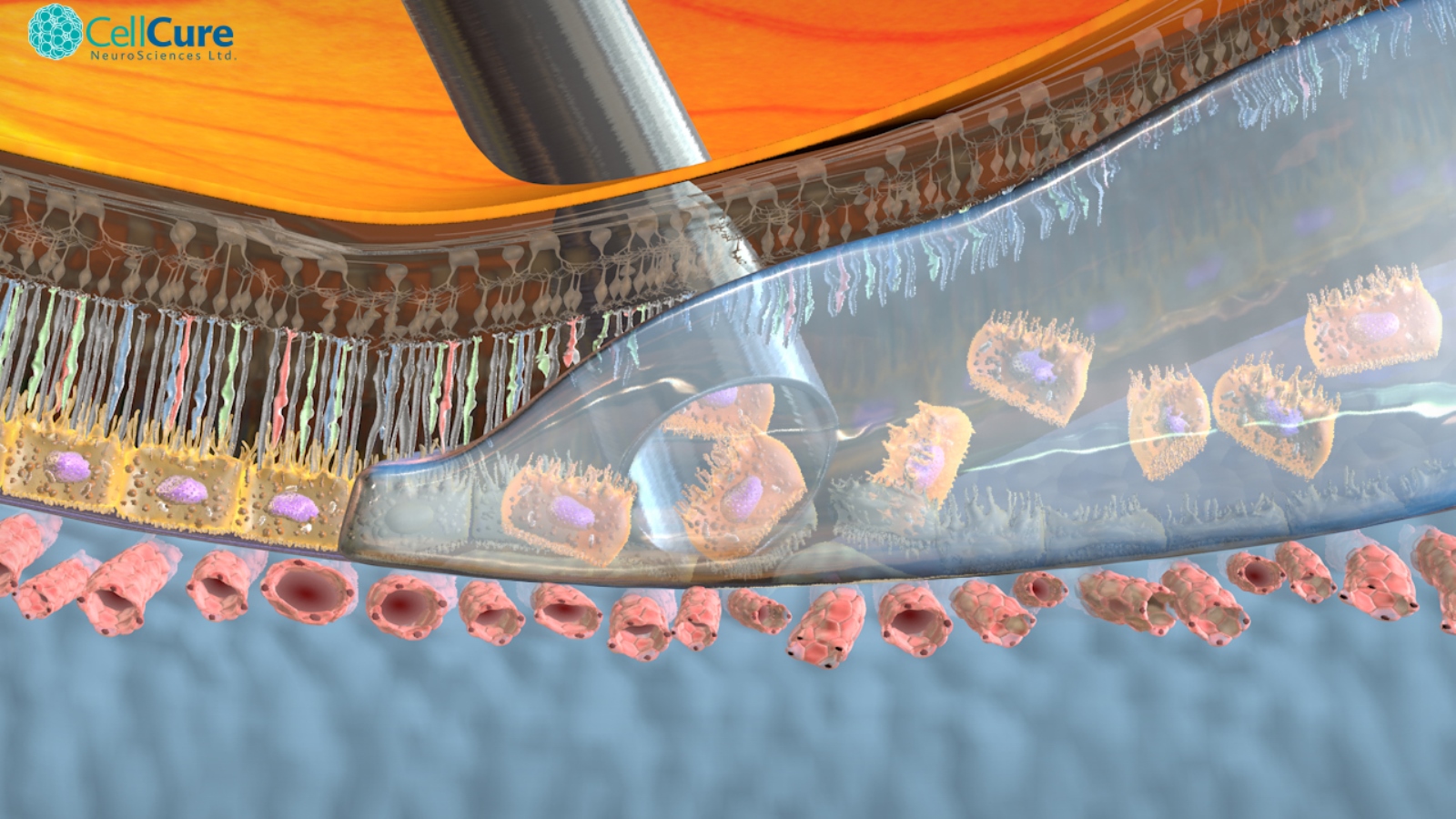
The more prevalent experimental approach is to organize the replacement RPE cells on a patch for implantation in a surgical procedure requiring special training. In contrast, OpRegen is injected as a suspension in a short, one-time procedure using the existing skill set of retinal surgeons.
Cell therapy has wider potential than do biologic treatments now being explored, says Irving. Pharmaceuticals such as monoclonal antibodies can only try to prolong the life of aging RPEs rather than replace them.
Still, Irving says, “We’re at the beginning of the race and there doesn’t have to be only one winner. There is no approved therapy right now so there is place for a few players.”
Seeking volunteers
Founded in 2005, Cell Cure has 25 employees. Chief Scientific Officer Dr. Benjamin Reubinoff was one of the first in the world to derive human embryonic stem cells and is an expert in their use in regenerative medicine. The company’s three main shareholders are US-based BioTime, Hadasit Bio-Holdings (a publicly traded subsidiary of the tech-transfer company of Hadassah) and Teva Pharmaceuticals.
OpRegen’s route to commercialization depends on the outcome of the current study and approvals for efficacy studies, as well as financing and business partnerships. Cell Cure has received annual grants totaling approximately $9.6 million from the Israel Innovation Authority (formerly the Office of the Chief Scientist) of the Ministry of Economy and Industry.
“Right now we want people to volunteer as participants in this or the upcoming Phase II study,” says Irving, who has a PhD in chemistry from the Weizmann Institute of Science in Rehovot.
Click here for information on trials, or email info@cellcure.co.il. Interested patients in Israel may contact Debbie Marks Ohana (devoramarks@gmail.com) or Itay Zafrany (etaihadassah@gmail.com) at Hadassah Ein Kerem.
Other Israeli solutions for AMD include ForeseeHome from Notal Vision, the first FDA-cleared home monitoring device to detect changes in vision that may indicate progression of AMD from the dry to the wet form; VisionCare Ophthalmic Technologies’ telescope implant for patients with end-stage AMD; and Nano-Retina’s artificial retinal prosthesis now being developed in collaboration with a nanotech lab in Texas.
For more information, click here.