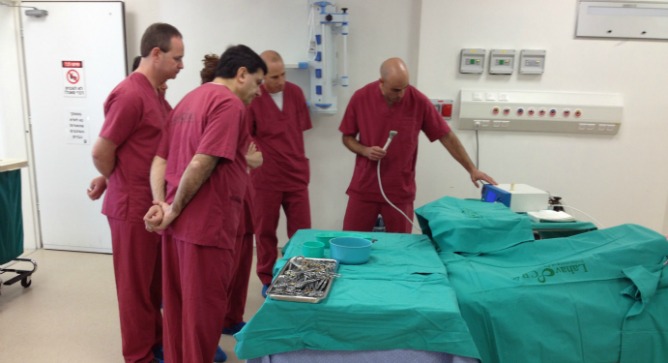
Israel’s IonMed has received CE Mark clearance for its Bioweld1 cold plasma system for surgical incision closure — the first-ever system in the rapidly emerging field of plasma medicine designed as an alternative to surgical staples, sutures and sealants.
“The use of cold plasma holds enormous promise as a new therapeutic paradigm in wound care, and more broadly in medicine,” said Shai Levanon, CEO of IonMed. “With the CE Mark clearance of Bioweld1, our first system, we have achieved important validation for our technology, a key milestone for the company, and a significant step towards commercialization.”
IonMed, a portfolio company of The Trendlines Group and of Generali Financial Holdings Fund, will seek a distribution partner to begin commercializing the system in Europe during 2015.
The CE Mark clearance was based on positive preclinical as well as clinical studies, including a trial of 20 women undergoing elective cesarean section and a pilot human evaluation of skin grafting. Other studies in preclinical models have documented safety, rapid disinfection, and efficacy in skin graft closure.
Fighting for Israel's truth
We cover what makes life in Israel so special — it's people. A non-profit organization, ISRAEL21c's team of journalists are committed to telling stories that humanize Israelis and show their positive impact on our world. You can bring these stories to life by making a donation of $6/month.