Scientists have managed to create synthetic human embryo models without using egg, sperm or womb, in a feat that could impact research on fertility, tissue growth and drug testing, as well as improve science’s understanding of the first weeks of embryonic development.
In the laboratory of Prof. Jacob Hanna at the Weizmann Institute of Science, researchers created complete models of human embryos from stem cells cultured in a lab grew them up to day 14.
While previous studies of cellular aggregates derived from human stem cells could not be considered accurate human embryo models because they lacked many of the defining characteristics of a post-implementation embryo, the Weizmann synthetic embryo models had all the structures characteristic of this stage, such as the placenta and yolk sac.
As described in Nature, the Hanna lab’s achievement could allow scientists to examine what is still considered an embryo’s mysterious beginning.
“The drama is in the first month. The remaining eight months of pregnancy are mainly lots of growth,” said Hanna. “But that first month is still largely a black box.”
“Our stem cell-derived human embryo model offers an ethical and accessible way of peering into this box. It closely mimics the development of a real human embryo, particularly the emergence of its exquisitely fine architecture.”
Unleashing potential
The researchers started out with human pluripotent stem cells, which can differentiate into various cell types. Some of these were derived from reprogrammed adult skin cells and others were the progeny of lab-cultured human stem-cell lines.
The researchers reprogrammed the pluripotent stem cells to an earlier (naïve) stage corresponding to day 7 of a natural human embryo, around the time it implants itself in the womb.
The cells were then divided into three groups. The first, which was meant to develop into the embryo, was left as is. The second and third groups, from which the placenta, yolk sac and chorionic sac were meant to develop, were treated with chemicals to turn on certain genes.
All three groups were mixed together and formed clumps, about 1 percent of which self-organized into complete embryo-like structures.
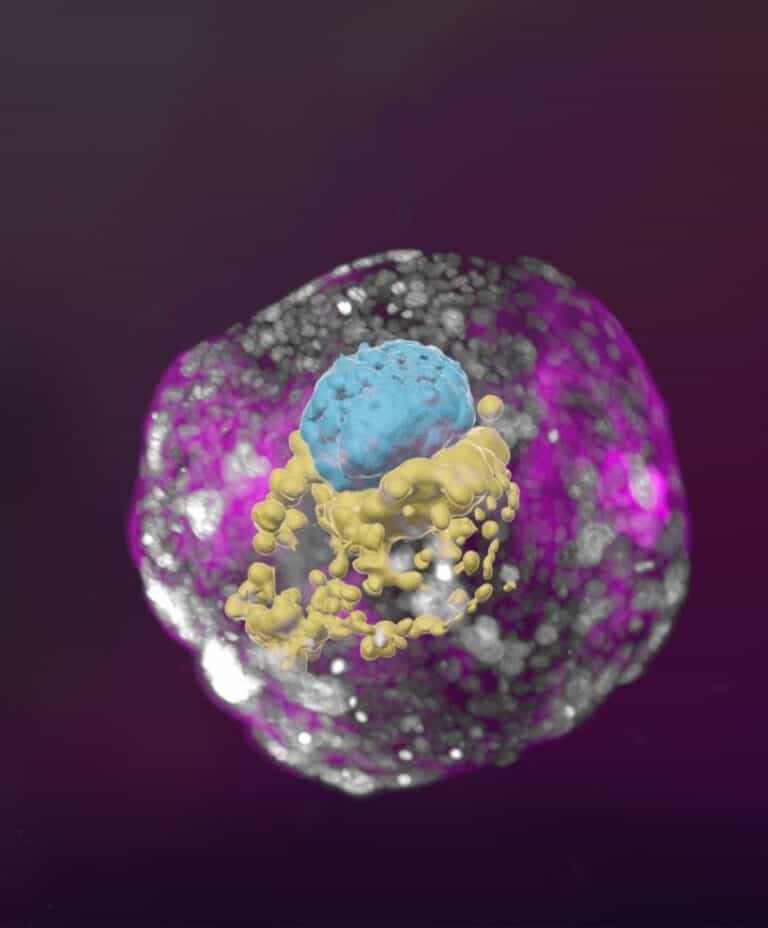
“An embryo is self-driven by definition; we don’t need to tell it what to do – we must only unleash its internally encoded potential,” Hanna said. “It’s critical to mix in the right kinds of cells at the beginning, which can only be derived from naïve stem cells that have no developmental restrictions. Once you do that, the embryo-like model itself says, ‘Go!’”
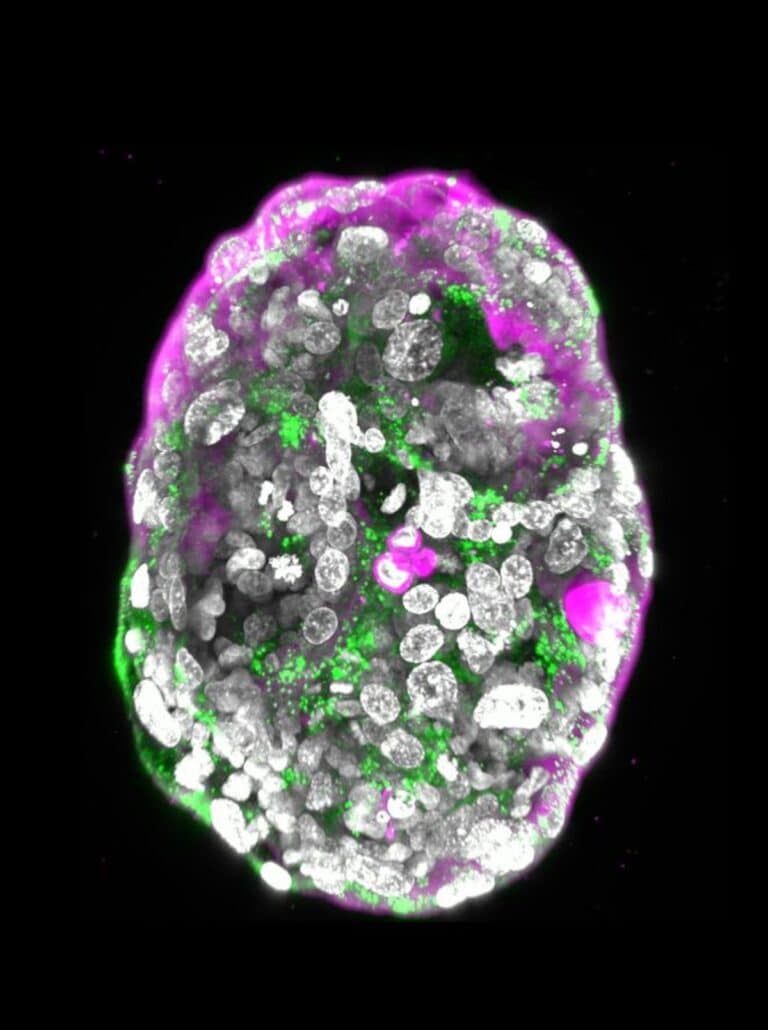
These embryo-like structures went on to develop for eight days outside the womb, reaching a stage equivalent to day 14 in natural human embryonic development.
Revealing what can go wrong
“An embryo is not static. It must have the right cells in the right organization, and it must be able to progress – it’s about being and becoming,” said Hanna, whose lab created mouse embryo models last year.
“Our complete embryo models will help researchers address the most basic questions about what determines its proper growth.”

Hanna noted that many failures of pregnancy occur in the first few weeks, often before the woman even knows she’s pregnant.
“That’s also when many birth defects originate, even though they tend to be discovered much later. Our models can be used to reveal the biochemical and mechanical signals that ensure proper development at this early stage, and the ways in which that development can go wrong.”
The synthetic, lab-grown embryo models could also offer a way around experiments that cannot be performed on live embryos, such as determining the effects of exposure to drugs or other substances on fetal development. Furthermore, it could lead to new technologies for growing transplant tissues and organs.















