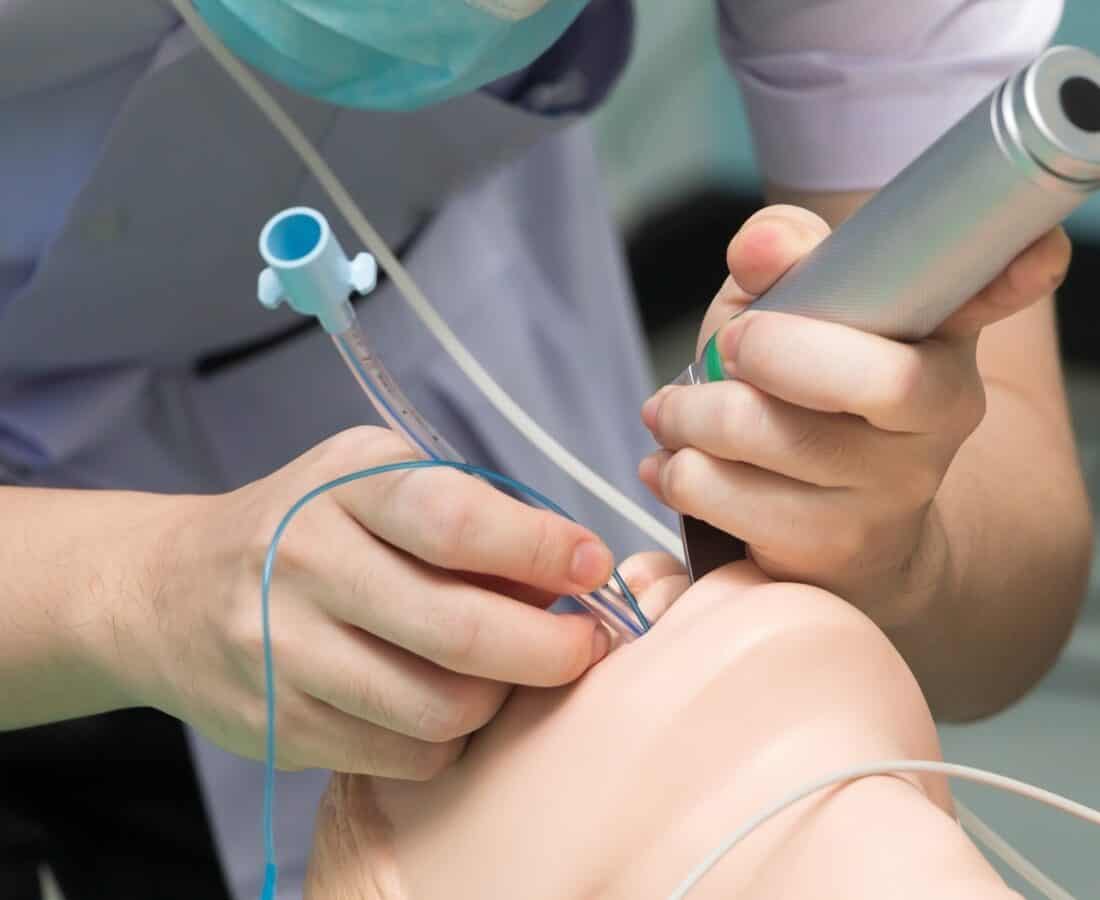
During the Covid-19 crisis, the medical term “intubation” entered common parlance.
Severe Covid patients, unable to breathe on their own, sometimes needed to have a tube inserted into their lungs hooked up to a mechanical ventilator. Intubated patients frequently are put into a medically induced coma.
Intubation and coma are terrifying for both patient and doctor. A significant proportion of patients do not survive. Millions of people around the globe suffer from acute respiratory failure from different causes – not just Covid – and 35.9% never make it out of the hospital.
Those who do survive face a long recovery period. Readmission rates are high and around 30 percent of patients develop PTSD.
Intubation doesn’t cure a severe lung infection. It simply gives the body time to respond to medication or to heal itself.
“If a patient’s blood oxygenation level continues to deteriorate despite non-invasive efforts, such as an oxygen mask, then invasive mechanical ventilation becomes the only option of treatment,” explains Prof. Benad Goldwasser, a urologist by training and chairman of the board of Inspira, a Ra’anana-based startup testing a less invasive concept for oxygenating blood without intubation.
Inspira’s unique Inspira ART500 (it stands for “Augmented Respiratory Technology”) system, still in the development phase, draws up to two liters of blood per minute into a portable device that removes excess CO2, enriches the blood with oxygen, and returns it to the patient via the same vein through a novel dual lumen cannula.
Think of it like a kidney dialysis machine but for blood oxygenation.
Even though the ART500 requires only a relatively small amount of blood (about one liter), the method can raise the oxygenation level of a patient to the “safe zone” of 95% in 60 seconds. Patients remain awake and breathing on their own.
Like working in the dark
Dagi Ben-Noon, a serial entrepreneur who previously cofounded Nano Dimension, established Inspira in 2018 with South African-born Joe Hayon, previously corporate controller and chief information officer for defense vehicle manufacturer Plassan Sasa. The two met in boot camp in the Golani division of the Israel Defense Forces.

“When we treat patients in a coma, it’s like working in the dark,” Hayon tells ISRAEL21c. “We can’t ask them how they’re feeling.”
Patients who require invasive mechanical ventilation are typically intubated for one to two weeks, which, Hayon says, is simply “courting problems.”
The longer a patient is mechanically ventilated, the more negative side effects develop – such as muscle loss and pressure wounds. Mechanical ventilators can overinflate the lungs, damaging the lung tissue. Furthermore, one out of nine patients develop “ventilator pneumonia,” Hayon adds.
This happened to Hayon’s wife’s grandfather. In the hospital for a checkup, he choked on some food and had to be put on a mechanical ventilator.
“Within a day, he developed pneumonia from the ventilator,” Hayon recalls. “He died a few days later.”

Hayon was sure there must be a better solution. “If you broke your arm, I wouldn’t ask you to pick up boxes. So, if your lung is sick or ‘broken,’ why provide such aggressive pressure through the lungs which makes them even sicker?”
He and Ben-Noon looked to nature for inspiration.
“Babies in the womb breathe without using their lungs. The oxygen goes directly through a membrane to the fetus,” Hayon tells ISRAEL21c.
“We thought, if we can bypass the sick lung like that, and go directly to the blood, we could create the same treatment conditions, but in grown-ups.”
Predictive analytics
The Inspira ART500 will have built-in blood sensing and AI capabilities.
“The system’s predictive analytics target is to monitor key parameters in the blood,” Hayon explains. “We can potentially know more about what’s happening with the patients and alert their doctors about any changes or abnormalities in real time and urge early intervention.”
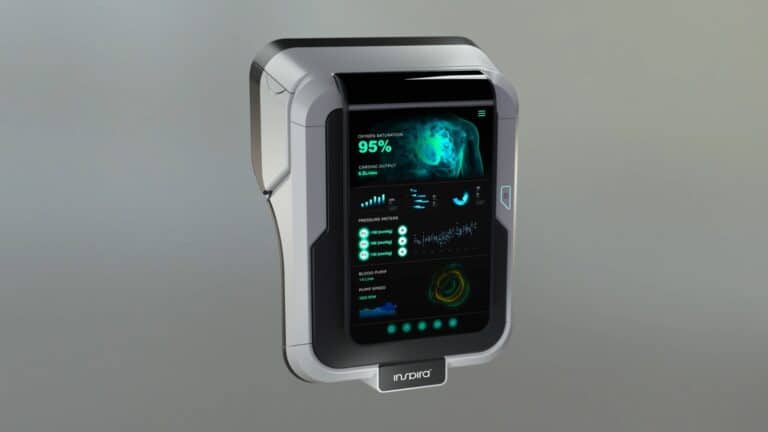
The ART500’s optical sensor is designed to deliver results “without the need for an invasive procedure or, actually, any physical contact with the blood at all,” Hayon adds.
Inspira’s device is expected to have benefits for hospitals and insurance companies, as well: By avoiding medically induced comas, hospital stays are shorter and there are fewer complications associated with mechanical ventilation, such as infections, lung injury and death.
Moreover, Hayon adds, the cost of caring for an intubated patient can run up to tens of thousands of dollars per week per patient. Using the ART500 could reduce hospital costs by up to 70%.
$20 billion market
Hayon estimates the total addressable market for Inspira’s ART500 and other non-invasive intubation alternatives on which the company is working at $20 billion.
Inspira has signed an agreement with Teruma Cardiovascular, a Japan-based company with a strong US presence, which develops heart-lung machines as well as oxygenation and monitoring systems. Teruma will supply a flow mechanism for Inspira’s blood circulation process.
The ART500 has not yet been tested or used in humans, so it will take time before it could be on the market. The aim is to begin a clinical pilot study in 2024 ahead of applying for regulatory clearance.
Meanwhile, the company has submitted its ART100 device, which provides cardio and pulmonary bypass support, to the FDA for clearance.
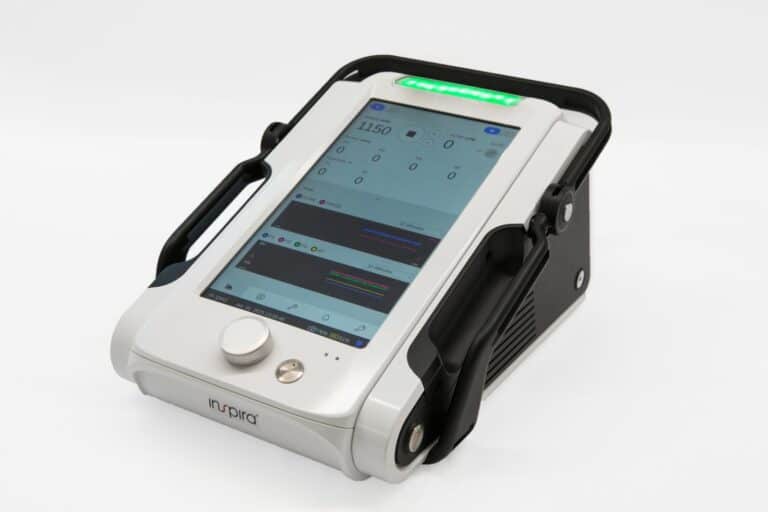
Hayon anticipates that Inspira’s business model will be similar to the printer and razor blade model, with the majority of revenue coming not from the main unit but rather from sales of disposable kits alongside software upgrade packages.
Inspira has raised $37.5 million and went public on NASDAQ in 2021. The company employs 50 people.
The name reflects both the hope that Inspira can be an inspiration for better health and a reminder to inhale, to breathe (inspiration).
For more about Inspira, click here.