Hearts can be broken in so many ways, and aren’t easy to fix.
We’re talking here not about emotional hearts but about physical hearts containing four chambers — left and right atriums on the top; left and right ventricles on the bottom – working together to circulate blood through our bodies.
Below we look at four Israeli startups with unique technologies for repairing or monitoring malfunctioning chambers on the left side of the heart.
First, a short anatomy lesson: The left atrium receives oxygenated blood returning from the lungs and pumps it into the left ventricle through the mitral valve. From there, the blood is ready to go on its bodily voyage.
Heart failure, affecting as many as 26 million adults worldwide, occurs gradually as the heart muscle weakens and struggles to do its job. The more common left-sided heart failure happens when the left ventricle doesn’t pump efficiently. Blood backs up into the lungs, causing shortness of breath and fluid buildup.
With that background, we’re ready to look at technologies focusing on left-sided heart failure.
Leviticus Cardio
Thousands of advanced heart-failure patients every year get an implanted mechanical pump called a left ventricular assist device (LVAD). The LVAD is connected to an external power pack by a wire (“driveline”) to charge the batteries and must be worn 24/7.
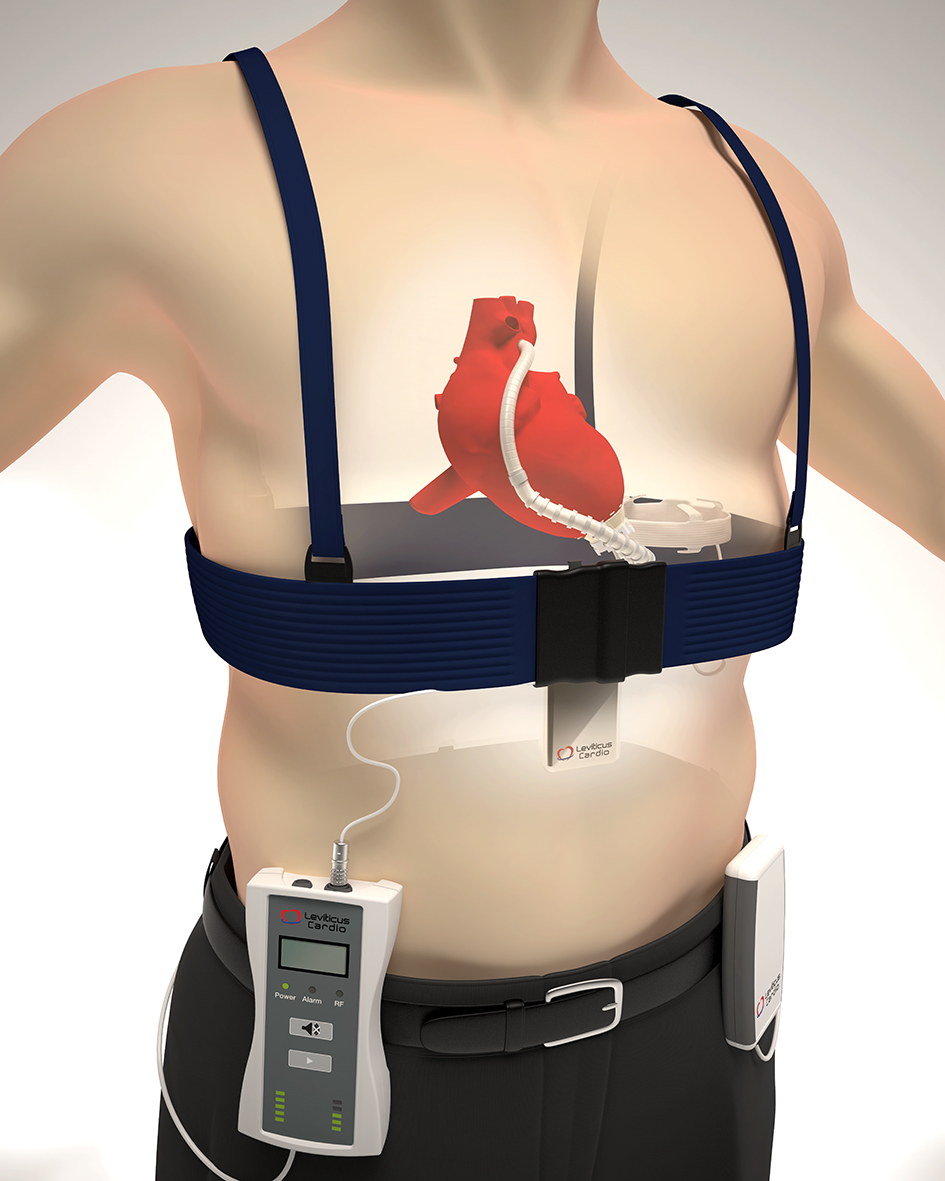
The driveline restricts mobility and is a source of infection and other potentially serious complications. Therefore, many patients who could benefit from LVAD therapy choose not to get implanted.
Leviticus Cardio has developed the only available wireless system for powering LVADs. The Coplanar Energy Transfer (CET) system makes it safer to receive an LVAD and allows patients to remove the external hardware for eight hours at a time.
In animal studies conducted at the Catholic University in Belgium, the CET system was shown to work efficiently for months, and it’s now ready for human trials.
Leviticus Cardio was founded in 2008 in Petah Tikva. Major investors include The Trendlines Group, medical and agricultural companies, a consortium of cardiovascular physicians, private investors and the Israel Innovation Authority.
CorAlert
Another Trendlines Group portfolio company, CorAlert is developing an accurate, low-cost, noninvasive detection and monitoring device measuring real-time left ventricular end-diastolic pressure (LVEDP), the standard way to detect and diagnose heart failure.
“LVEDP is always elevated in heart-failure patients and is elevated days or weeks before a severe condition appears, so it’s very predictive for the prognosis of the disease,” explains CorAlert CEO Amir Marmor, a software engineer who founded the company with his father, Dr. Alon Marmor, professor of cardiology at Bar-Ilan University and former director of cardiology at Ziv Medical Center in Safed (Tzfat).
Existing methods for measuring LVEDP are via a cardiac catheter or estimating pressure from Doppler echocardiography. Both have major drawbacks, and CorAlert aims to provide an alternative that cardiologists have been seeking for years, says Marmor.
CorAlert’s system consists of two sensors: one placed on the chest and one on the arm inside a smart compression (blood pressure) cuff controlled by proprietary software that adjusts the pump to the patient’s heart rate.
The sensors provide data on heartbeat and pressure from which LVEDP can be accurately estimated using a unique mathematical formula.
The beta model of the system is starting clinical trials at Ziv and at UC San Francisco Medical Center. “We will compare LVEDP measured by our device to invasively measured LVEDP and validate the accuracy of our device,” says Marmor.
Founded in 2012 in Ramat Hasharon, CorAlert is now raising $2 million.
Vectorious Medical Technologies
Moving up from the ventricle to the atrium, Tel Aviv-based Vectorious Medical Technologies is developing V-LAP, the world’s first miniature wireless microcomputer implant for left-atrial monitoring of heart failure.
The company recently raised $9.5 million, plus a $2 million Horizon 2020 grant from the EU Research and Innovation Program, as it increases staffing and prepares for clinical trials leading to EU regulatory approval.
V-LAP transmits daily pressure readings to the physician, who can easily spot any changes and adjust medication accordingly to prevent deterioration – something not currently possible in real time. The system will include an external home unit for the patient to self-monitor changes as well.
Founded in 2011 in the RadBioMed incubator, Vectorious has 15 employees in Tel Aviv and at the Cleveland Clinic, one of the company’s investors.
“Chronic lack of supply to the heart is one of the most common causes of death in the Western world — 1.2 million people are hospitalized each year in the US from deterioration of the disease at a cost of $32 billion to the US health system,” Vectorious CEO Oren Goldstein said.
“In addition, half of patients need to be re-hospitalized after six months because of defective diagnosis and treatment. Vectorious’s product will prevent the disease from worsening, improve the quality of life of the patient and lengthen their lives.”
V-Wave
Caesarea-based V-Wave recently got a $70 million Series C investment that will help it move forward with clinical trials in North America, Europe and Israel of its V-Wave Interatrial Shunt System — a miniature, biocompatible interatrial implant to regulate left atrial pressure (LAP) in patients with chronic heart failure.
The shunt is intended to prevent episodes of elevated LAP and is thus expected to provide symptom relief, decrease hospitalization, increase exercise tolerance, improve quality of life and perhaps even extend survival.
Dr. William T. Abraham, chief of cardiovascular medicine at the Ohio State University Wexner Medical Center, explained that elevated LAP is the primary cause of breathing difficulty and hospitalization due to worsening heart failure.
“V-Wave’s feasibility study results, presented in March 2018 as a late-breaking clinical trial at the American College of Cardiology, showed that shunting was safe and that morbidity and mortality were low compared to a matched population receiving optimal care. The upcoming pivotal trial in at least 400 randomized patients should provide sound assurance of the efficacy of this approach in patients that have a poor prognosis and few options,” said Abraham.