If you’re among the 10 percent of the world’s population unfortunate enough to suffer from migraine headaches, you know only too well the sensation of severe throbbing or pulsing pain often accompanied by nausea, vomiting, dizziness and extreme sensitivity to light and sound.
These uber-headaches can be so debilitating that even after taking over-the-counter medications you may just need to crawl into bed in a dark room and skip whatever it was you were supposed to do that day.
It’s no wonder that people who get migraines – considered the third most prevalent illness in the world — are more likely to suffer also from anxiety, depression and sleep disturbances, according to the US-based Migraine Research Foundation.
Various out-of-the-box thinkers in Israel have been seeking better ways to banish migraine headaches. Here are a few approaches in various stages of development.
- Netanya-based Theranica Bioelectronics developed the Nerivio Migra neuromodulation patch, recently cleared by the US Food & Drug Administration.
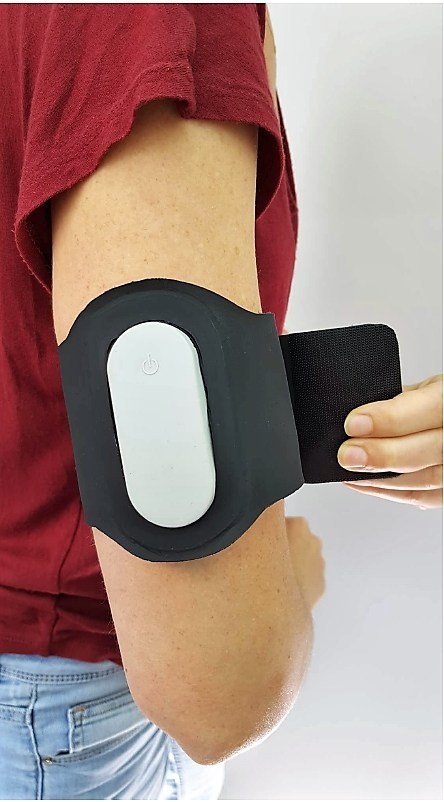
Tiny electrodes inside thebattery-powered patch deliver electrical nerve stimulation to reduce the perception of pain from the migraine. Worn on the arm, Nerivio Migra communicates wirelessly via Bluetooth with your smartphone, which controls the electrical pulses and length of treatment.
In Theranica’s clinical trials in the US and Israel, about two-thirds of participants reported significant pain relief and 30 percent said they were pain free, although the effect is temporary. The company says a 45-minute treatment can bring relief for up to two days.
Nerivio Migra will be available in limited quantities in late 2019, with wider distribution beginning in early 2020.
The device is indicated for acute treatment of migraine with or without aura in adult patients who do not have chronic migraine. (The aura sensation, which temporarily affects vision, coordination and speech, occurs in about 20% of people with recurring migraines.)
- Neurolief, also headquartered in Netanya, recently received the European CE mark of approval for its Relivion head-mounted neuromodulation device for migraine pain and relief of depression, ADHD and insomnia.
Relivion uniquely offers pain-blocking occipital (back of the head) and trigeminal (facial) nerve stimulation noninvasively, at home, without a prescription. In a randomized, double-blind, placebo-controlled clinical trial last year, 76% of participants achieved migraine headache relief after only one Relivion treatment.
The rechargeable neuromodulator stimulates six nerve branches that regulate pain and mood, preventing the secretion of brain chemicals that trigger pain and modulating the “activation threshold” of the neural system to lessen the future effects of migraine triggers such as stress, chocolate, cheese or wine.
The folding portable Relivion connects to a mobile phone app and cloud-based analytics to determine the most effective stimulation for each user. The device is sold pre-loaded with 10 treatments; users can load more remotely via the Internet.
- Teva Pharmaceuticals of Jerusalem received both FDA and CE approval for Ajovy (fremanezumab), an injectable migraine-prevention medication administered monthly or quarterly in adults who have at least four migraine days per month. About 40% of migraine sufferers may be candidates for Ajovy.
In clinical trials, many patients on Ajovy experienced reductions of at least 50% in the number of monthly migraine days, starting as early as week one. Ajovy can be administered by a healthcare professional or at home by a trained patient or caregiver.
- More than a decade ago, Israeli neurologist Dr. Yaron River developed Occiflex, a robotic device for physical therapists to treat neck and head pain. River says, “The device is not directly intended for the treatment of migraine. However, in chronic migraine, associated neck pain could be relieved and consequently, migraine severity and frequency [diminished].”
The patient’s head and neck rest in an adjustable computer-guided cradle attached to a customized treatment table. The cradle moves the head gently along a predefined three-dimensional path, relaxing the muscles to reduce pain in a personalized 20-minute session set by the therapist and stored in the computer for subsequent automated sessions. Treatment usually lasts three times a week for a month or two.
The system was developed and commercialized by Headway Medical in conjunction with Dutch medical rehabilitation and physiotherapy device manufacturer Enraf-Nonius, which exclusively distributes Occiflex in Europe. The treatment is also available in Israel; FDA approval has not yet been granted for US sales.
- A potential pharmaceutical therapy for neurological disorders including migraine headaches recently was revealed by BGN Technologies, the technology-transfer company of Ben-Gurion University of the Negev.

The regimen combines two FDA-approved drugs, Memantine and Losartan, which protect the blood-brain barrier (BBB), therefore preventing the development of various neurological conditions.
BBB malfunction is a major factor in neurological diseases including stroke, neurodegenerative diseases, brain tumors and brain infections. Preclinical studies show the combination treatment protects the integrity of the BBB. Partners are currently sought by BGN to further development of this promising combination therapy.















