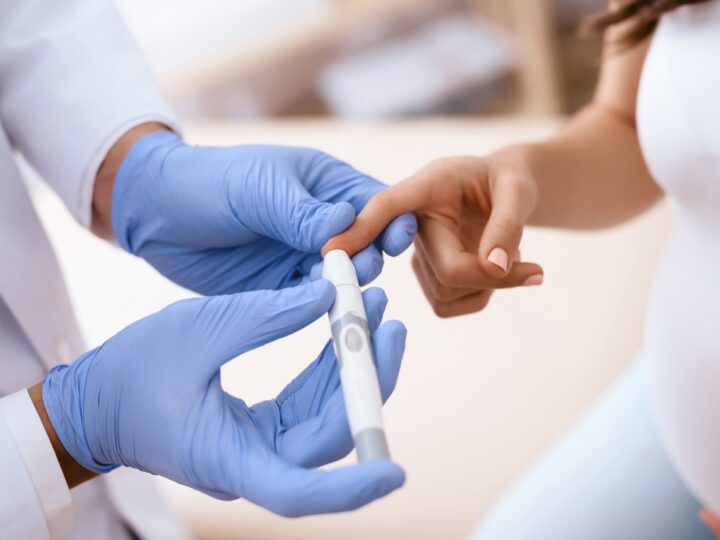
November 14 is World Diabetes Day and the month of November is National Diabetes Month in the United States. While there is not yet a cure for diabetes, many Israeli researchers and companies offer improved approaches for avoiding, managing and treating the condition.
Diabetes is a chronic disease that occurs when the pancreas cannot make insulin – the hormone that regulates blood sugar — or when the body cannot make good use of the insulin it makes.
As of 2015, approximately 415 million adults in the world have diabetes and that number is expected to rise to 642 million by 2040. At least 90 percent of cases are type 2 diabetes, characterized by insulin resistance and/or deficiency. In addition, more than 542,000 children in the world live with type 1 diabetes, an autoimmune condition where the body attacks its own insulin-making cells.
Diabetes is the leading cause of heart disease, stroke, kidney failure, non-traumatic lower-limb amputations and new cases of blindness among adults. Those with diabetes have twice the normal risk of death; in 2014, diabetes was listed as the seventh leading cause of death in the United States.
Here are some significant diabetes developments reported in Israel in recent years.
Oramed Pharmaceuticals hopes to revolutionize the treatment of type 1 and type 2 diabetes through its proprietary oral insulin capsule developed through research at Jerusalem’s Hadassah Medical Center. Currently, insulin must be injected.
The company has completed multiple Phase II clinical trials under an Investigational New Drug application with the US Food and Drug Administration. Oramed CEO Nadav Kidron participated in the 2017 Disruptive Growth Company Showcase NYC Conference in September in New York City.
Dario makes a personalized, pocket-sized, all-in-one glucose meter coupled with a real-time mobile app to track, monitor and manage diabetes from a smartphone.
Last month, this Caesarea-based digital health company received the CE Mark for its Lightning-enabled Dario Blood Glucose Monitoring System, which will enable European consumers to use the metering device on the latest Apple devices. The regulatory process is now starting in the US, Canada and Australia. The company’s new B2B2C platform, Dario Engage, helps healthcare providers in all aspects of user engagement for diabetic patients, including enrollment, coaching and ongoing communication.
Betalin is developing an implantable engineered micro-pancreas (EMP) using a natural micro-scaffold and all the cellular components present in a natural pancreas. These cells reconstruct the body’s internal insulin-producing capability in accordance with the blood-sugar levels. Type 1 and severe type 2 diabetes patients implanted with the micro-pancreas would no longer need to monitor their blood-sugar levels.
In preclinical studies in a mouse model of diabetes, approximately 70% of the mice in which the Betalin EMP was implanted did not need further insulin injections even for the longest period tested of 90 days after implantation.
This early-stage company, spun out of the Hebrew University of Jerusalem, won in the pharmaceuticals category at the Biomed Startup of the Year competition held by the Israel Innovation Authority at MIXii Biomed 2017 in Tel Aviv last May.
- GlucoTrack

Integrity Applications of Ashdod put more than a decade into developing GlucoTrack, a revolutionary noninvasive system for self-monitoring glucose levels, available so far in several European countries, South Korea and Israel.
The GlucoTrack sensor clips onto your earlobe. A patented combination of ultrasonic, electromagnetic and thermal technologies works with a proprietary algorithm to measure physiological parameters correlated with glucose level. Results are displayed within about a minute on a USB-connected handheld control unit, which also stores and compares previous readings. The number is announced verbally, facilitating use by elderly and vision-impaired people with type 2 diabetes or pre-diabetes.
Another Israeli company, Cnoga Medical, offers a prick-free glucometer that uses a camera to observe changing color shades of the user’s finger to give accurate results comparable to those of a fingerprick, following a short “training” period in which the device learns to correlate the user’s optical skin-tone characteristics with camera readings. The COG device is certified in Israel, Europe, New Zealand, Turkey, India, Philippines, Thailand, Brazil and China. A new clinical study is starting in Sao Paolo.

The Sweetch app, now in clinical trials at Johns Hopkins Medical Center in Maryland, predicts personal diabetes risk and encourages long-term behavioral change to prevent diabetes. According to the Tel Aviv-based company, 79 million American and 63 million EU adults are pre-diabetic; 70% of these will convert to diabetes within a decade. However, 150 active minutes per week has proven, in large clinical trials, to reduce pre-diabetes to diabetes conversion by 58%.
“We see very promising initial meaningful clinical results in all three aspects — increased activity, weight loss and A1C [average blood-sugar level over three months] reduction,” Sweetch CEO Dana Chanan tells ISRAEL21c.
Sweetch is talking with healthcare stakeholders in the United States and United Kingdom regarding future implementation of the app.
Many diabetics manually log their insulin injections to avoid over- or under-dosing. Insulog is a small device that snaps onto all major brands of disposable insulin pens to display the user’s most recent data.
Insulog’s smartphone app uses Bluetooth technology to store injection history, including number of units administered, and blood-glucose level. Users can share this history with their physician.
The company was founded in 2014 in Ramat Gan by Menash Michael, who has type 1 diabetes. He is developing the device for delivery in 2018 following a successful Indiegogo campaign earlier this year. It needs only Class 1 FDA approval with no required clinical trials. “We will market Insulog first in Israel and then on an ecommerce platform,” he tells ISRAEL21c.
https://www.youtube.com/watch?v=3wsmi6_E7A8&feature=youtu.be
Based in Petah Tikva, DreaMed has two products: Advisor decision-support technology to optimize and fine-tune patient-specific insulin treatment plans (FDA and CE approval pending); and Glucositter, the first artificial pancreas system to receive the CE Mark for sales in Europe. When integrated with an insulin pump, Glucositter provides round-the-clock monitoring of glucose levels and precise, real-time adjustment of insulin levels.
Nutrino Health, the digital healthcare Biomed Startup of the Year winner at MIXii 2017, has developed an app (for iOS and Android) that creates a digital individualized FoodPrint using data from the user’s medical devices, wearables, activity sensors and other biological markers.
Taking into account personal parameters such as allergies, food preferences and more, the system offers menus and foods especially adapted to the user, and helps diabetics understand how food affects the management of glucose levels in their blood.
The free version of the app allows users to log food, exercise and medication and track these measures according to goals, taste preferences and dietary needs. They also receive access to personalized daily health tips. Premium features include a personalized meal planner, custom diet options, full recipe browser, suggestions on healthy dishes from nearby restaurants.
DayTwo, based on groundbreaking research at Israel’s Weizmann Institute of Science, aims to help people avoid developing diabetes by predicting individualized blood-glucose response to thousands of different foods and meals based on gut microbiome analysis and other personal parameters.
This year, DayTwo and the Weizmann Institute scientists collaborated with Johnson & Johnson’s Janssen Research & Development Company to evaluate DayTwo’s platform for the effective interception of gestational diabetes, type 2 diabetes and metabolic syndrome-associated disorders. This resulted in a Series A investment of $12 million led by J&J.
DayTwo currently is running trials with the Mayo Clinic and other institutions, while the product is fully commercial in Israel and US. International Chief Commercial Officer Amir Golan tells ISRAEL21c that the UK and Canada are the next potential markets. Meanwhile, the company is working with professional athletes to control blood-sugar levels and is partnering with two diabetes medical centers in Israel as well as dieticians in Israel and the US.
https://youtu.be/ET6ypQH4IIk
Researchers at Ben-Gurion University of the Negev have engineered a “super enzyme” that, if commercialized, could make blood-glucose checks easier and more accurate than the standard method of mixing a protein with a drop of blood to cause a chemical reaction. The research was reported in the Journal of the American Chemical Society in September 2017.