
The well-known problem in cancer radiation therapy is that it indiscriminately zaps both malignant and healthy tissues. But what if it were possible to shield normal cells from the powerful rays?
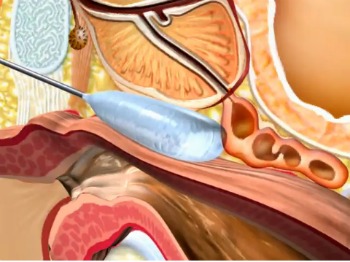
BioProtect, one of a cluster of notable Israeli biotech innovators, has come up with a unique solution that accomplishes this task for prostate cancer sufferers. Its ProSpace biodegradable balloon keeps radiation from healthy tissues surrounding the tumor.
This type of cancer was a logical place to start because of the prostate’s proximity to the rectum. Radiation therapy for the cancer can inadvertently cause soreness, diarrhea, perforation, and severe bleeding or bloody discharge.
“It’s a quality of life issue,” BioProtect CEO Amir Shiner tells Israel21c, noting that radiation therapy can also result in health conditions that require significant interventions. “There’s a real need for such a protection device.”
Made from biodegradable polymers, the balloon is implanted in a simple, minimally invasive procedure and dissolves within three to six months.
Nearly 50 percent of cancer patients in the United States are treated using radiation therapy, and ProSpace may have a role in offering many of them a safer and more enhanced radiation therapy experience.
According to the American Cancer Society, approximately 12 million new cases of cancer are diagnosed globally each year, and the incidence of cancer is expected to rise in the coming years.
Soft launch
BioProtect is aiming its breakthrough technology at the European market first. In October 2010, the device received the CE Mark, which certifies that a product conforms with European regulatory requirements. The company is now establishing its marketing network throughout the continent.
Shiner calls it a “soft launch process.”
“We already have initial sales in Europe. We started in Italy in April, and have since signed distribution agreements in five European countries. Right now, we’re working to validate the product’s position in the market and are negotiating to expand further in Europe.”
In Israel, the Health Ministry approved the ProSpace for marketing last July.
The company has already carried out a pilot study for the US Food & Drug Administration, and is now in the process of finalizing the clinical requirements to obtain clearance to sell ProSpace in the US.
Researching, developing and marketing a breakthrough technology requires deep pockets. Three groups of investors are backing BioProtect: Virginia Life Science Investments, a major investor in Israeli healthcare companies; the JumpStart NJ Angel Network; and Xenia Venture Capital, operator of the Kiryat Gat technological incubator that nurtured the company as a startup. There also several private investors.
“Right now, we’re working on more significant fundraising to take us to the next stage,” says Shiner. “We’re a small company, based in Kfar Saba, with a highly dedicated R&D team. Manufacturing is on site.”
Proven technology
After completing preclinical studies, BioProtect recruited 24 patients for its multi-center pivotal study for the European market, which involved VCU Massey Cancer Center and VA Urology Medical Centers in Virginia, the Montefiore Medical Center in New York City, Luebeck University Hospital of Schleswig-Holstein in Germany, the Padova University Hospital in Italy and Tel Aviv’s Sourasky Medical Center.
In March this year, Padova Hospital urologist Fabrizio Dal Moro reported to the European Association of Urology’s 26th Annual Congress that inserting ProSpace between the prostate and rectum has been shown to reduce the volume of rectum exposed to radiation by 69.9%, which he called “a dramatic reduction.”
In January next year, the company is to launch a further study utilizing ProSpace for an innovative radiation therapy protocol at several Israeli medical centers.
Shiner says that the feedback from the field has been extremely encouraging.
“Physicians tell us that they prefer our product to the only alternative available — a gel that is injected and performs a similar role. The great advantage of the ProSpace device is that it’s reproducible. Its well-defined boundaries under a CT scan, and reproducible shape and size, help the doctor to effectively administer their treatment.”
What’s more, patients report little or no pain from the implanted balloon, although they occasionally experience mild and temporary discomfort at the access area.
There are other advantages, Shiner notes: ProSpace enables more efficient treatments using more concentrated radiation doses, meaning fewer treatment sessions at a lower cost.















