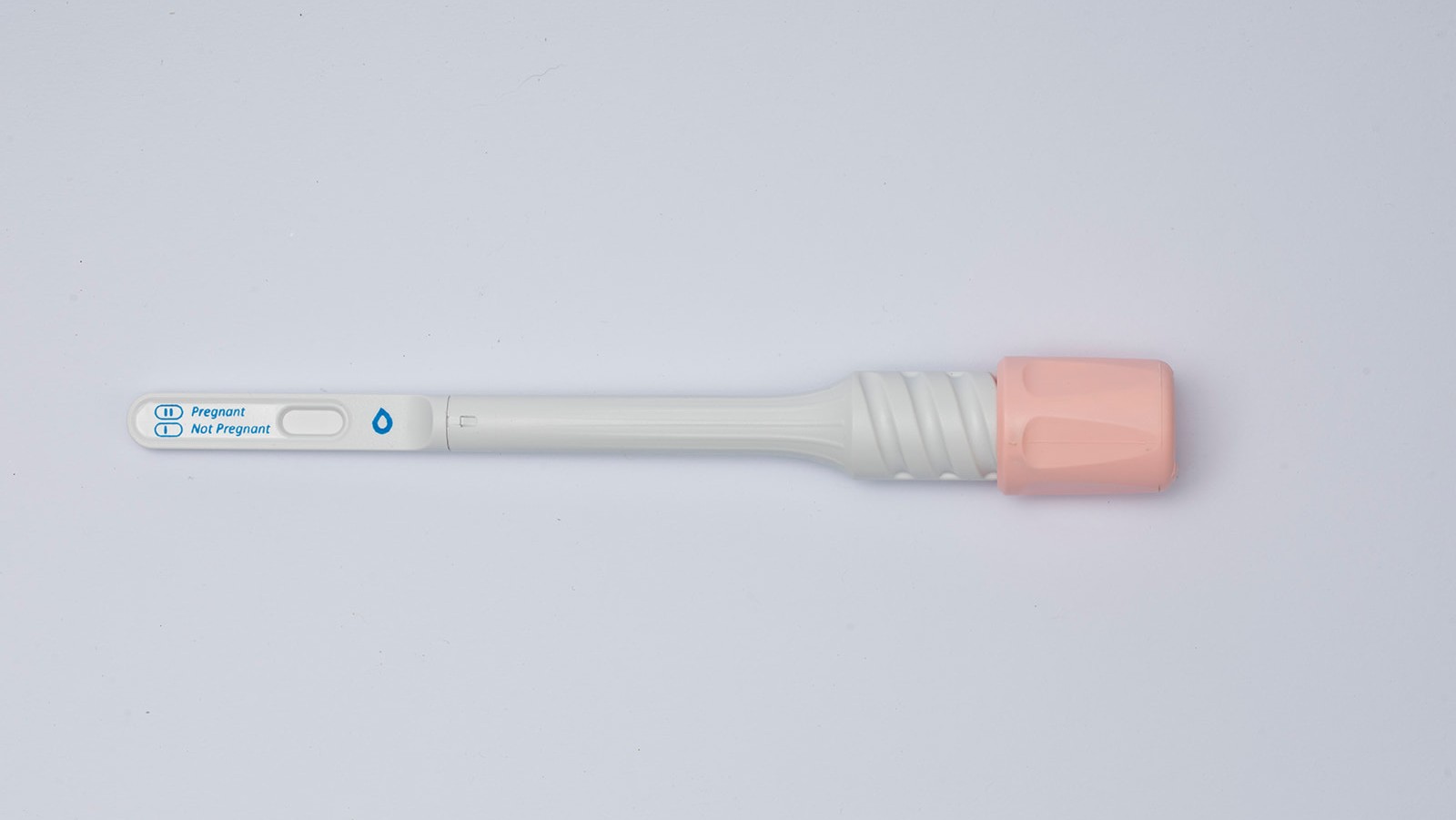
Peeing on a stick will soon be as obsolete as the “rabbit test” thanks to SaliStick, a revolutionary saliva-based rapid pregnancy test kit.
The product has successfully completed clinical trials and thousands of analytical trials in Israel and is expected to receive the European Union’s CE Mark soon. Approval by the US FDA could come next year.
SaliStick was developed by Jerusalem-based Salignostics based on its proprietary saliva-based hormone detection technology used in the company’s SaliCov rapid antigen saliva test kit to detect Covid-19, used widely in Europe and Africa.
SaliStick detects the pregnancy hormone β-hCG as early as the first day of a missed period.
https://www.youtube.com/watch?v=pwlBzpHrkC8
“Saliva is the key to rapid diagnostics for a variety of medical reasons. Quintessentially it is the only noninvasive, easy, and hygienic means to detect hormones, viruses and even diseases,” said Guy Krief, Salignostics’ cofounder, deputy CEO and director of business development.
“We are delivering a product that completely removes the need for blood and urine samples when testing for pregnancy,” he said. “With SaliStick, we hope to empower women by making the discovery of pregnancy more dignified and inclusive.”
The company will display the new product at the Medica 2021 exhibition in Düsseldorf, Germany, this week.
Salignostics was founded in 2016 by Krief and four other PhDs: Prof. Aaron Palmon, former dean of Hebrew University’s dental school; Omer Deutsch; Yoav Neumann; and Raluca Cohen.