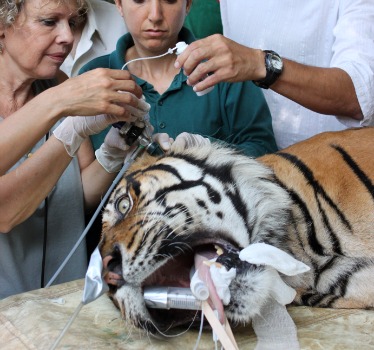
Pedang, a Sumatran tiger at the Ramat Gan Safari in Israel, has suffered from recurring ear infections over the past few years. Lately, the infections have become more frequent. So the zoological park turned to Rehovot-based Otic Pharma, which is developing a proprietary foam drug-delivery platform specifically for ears.
The company agreed to supply its unique experimental FoamOtic therapeutic solution to see if it can help cure Pedang once and for all.
Spread the Word
• Email this article to friends or colleagues
• Share this article on Facebook or Twitter
• Write about and link to this article on your blog
• Local relevancy? Send this article to your local press
Otic Pharma loaded its foam with a concoction of antibiotics, steroids and anti-fungal ingredients aimed at wiping out the microbes causing the disease, and reducing the underlying inflammation that is the likely cause of the tiger’s recurrent infections.
Pedang received the one-time treatment late last week and responded well, according to Otic Pharma Chief Operating Officer Rodrigo Yelin, a biochemist. Time will tell if the infections do not come back.
“One of FoamOtic’s main strengths is that, in contrast to eardrops, it does not spill out of the ear, despite the head-shaking that follows application of ear medications to animals,” Yelin says.
“Our preliminary studies show that [the foam] is well tolerated by dogs, and we are optimistic that our innovative treatment will be able to alleviate Pedang’s recurrent infections,” he adds.
The zoo staff shares his optimism.
“We believe that Otic Pharma’s novel treatment has the potential to significantly contribute to the tiger’s recovery,” said Dr. Igal Horowitz, Safari veterinarian and CEO of the Israeli Wildlife Hospital. To enhance the effects of the medication, he has also ordered complementary treatment with methods such as acupuncture.
For dogs, cats and humans, too
The bacterial species plaguing the tiger’s ears, Pseudomonas, is the same one that was successfully eradicated by Otic Pharma’s medication in clinical studies in human patients diagnosed with acute otitis externa (“swimmer’s ear”).
“We are convinced that our [foam], which is in development for the treatment of common ear disorders in humans, is also very well suited for dogs and cats, which frequently suffer from otitis externa,” Yelin says.
Dogs with earaches account for 14 million visits to the vet every year in the United States alone, according to Otic’s market research. However, the eardrops commonly prescribed are difficult for pet owners to administer. And a lot of the liquid drips right out of the ear when the animals shake their heads afterward.
FoamOtic is meant to address this problem with a foam drug-delivery platform that can incorporate a variety of active ingredients. The drugs are continuously released as the foam expands in the ear canal and coats it, with no need for keeping the head tilted to one side. Because none is lost through dripping, the medication has a longer-lasting effect and only requires a single dose.
Better still, says Yelin, the owner doesn’t have to do a thing. “This can be applied right after diagnosis by the veterinarian,” he says.
A successful study on dogs was completed in Israel. “We are starting development of the veterinary product and we’re looking for a large partner that can take the lead in co-development and marketing,” says Yelin. “I’m sure that it will reach the market and make a significant impact because giving medications is a really big issue for owners and we can spare them from that.”
Dr. Aya Peri, head veterinarian at the Society for Prevention of Cruelty to Animals in Israel, released a statement saying: “From my experience, FoamOtic is well tolerated by dogs, and I am confident that a single application of the product will be effective for the treatment of canine otitis externa. I am therefore hopeful that it will also be effective for the treatment of the ailing tiger.”
Meanwhile, Otic Pharma is launching a human study in Israel this summer – prime time for swimmer’s ear — to test its foam as a once-daily drug-delivery platform against the leading twice-daily eardrops on the world market, in both children and adults, for a treatment period of one week.
Because the FoamOtic platform utilizes drugs and ingredients already approved for use in the ear, the company expects the development and regulatory processes to be relatively fast.
The next product in the company’s pipeline is intended for treating middle ear and inner ear infections using the same delivery platform.