About 40 million epidural pain-relief injections are given annually worldwide, often administered to women in labor.
An epidural involves injecting an anesthetic or a steroid into the space around the spinal nerves to stop pain signals from the spine reaching the brain.
However, in 10-30 percent of cases the needle misses the correct spot on the first try.
And in up to 5% of cases, the needle punctures the protective sac around the spinal fluid, leading to severe headaches and even neurological complications.
Lior Margalit’s father was one of the unlucky ones.
“My father broke his leg when he was in South America on a business trip and needed orthopedic surgery. Although the surgery went well, he suffered side effects from the placement of the needle during the epidural,” says Margalit.
He decided to put his training as a biomedical engineer to the task.
Margalit spoke with many anesthesiologists and researched the problems involved in epidural injections before cofounding Omeq Medical more than 10 years ago.
Now, Omeq’s EpiFinder device has received regulatory clearance from the US Food and Drug Administration and will start being marketed early next year in the United States to assist physicians in accurately positioning epidural needles.
Traditional and trailblazing
Unlike some other solutions on the market for this purpose, EpiFinder was designed to enhance, rather than replace, the method doctors already use.
“Ours is the only real-time, objective indication for positioning that still allows doctors to use the standard technique,” Margalit tells ISRAEL21c.
Since the 1920s, epidurals have been performed utilizing the loss of resistance (LOR) technique. The anesthesiologist advances the epidural needle forward until sensing a loss of resistance indicating that the needle reached the narrow epidural space in the spine.
The success of LOR depends on the clinician’s experience, dexterity and tactile feedback.
“Doctors understand that they’re using a very subjective technique and they miss from time to time and need to solve that problem,” Margalit learned from his early conversations with anesthesiologists.
“But they wanted us to preserve their own technique at the same time. So we found a balance between tradition and innovation.”
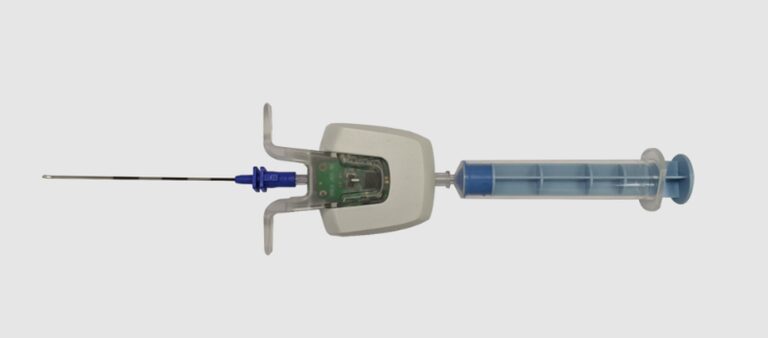
Instead of introducing a new technique or adding complexity to this already complex procedure, Omeq aims to improve LOR success with a battery-operated, sensor-based device placed between the needle and the syringe holding the medication.
EpiFinder sends a signal confirming when the needle tip has successfully reached the epidural space.
Will they use it?
In a clinical study of EpiFinder involving 31 Israeli patients, physicians delivered the epidural accurately every time, demonstrating the device’s safety and effectiveness.
The participating doctors gave Omeq valuable feedback on refining the device and expressed their satisfaction with EpiFinder’s ease of use and benefits.
This is critical, Margalit points out, in assuring the device will be adopted in what is estimated to be a $1 billon market.
“Clinical literature shows that a significant percentage of all epidural insertions are initially misplaced,” said Omeq’s medical director, Dr. David Gichtin, senior anesthesiologist at Baltimore’s Johns Hopkins Hospital.
“While most of these are corrected procedurally without adverse events, a meaningful number of complications … result in patient suffering and substantial institutional costs,’ Gichtin said.
“The EpiFinder smart device offers to improve the performance of epidural technique for potentially better outcomes, with the intent to make epidural procedures safer and easier for doctors and patients.”
Series A starting
A portfolio company of The Trendlines Group, Omeq Medical has been supported by Israeli Innovation Authority grants and is beginning a Series A funding round toward commercialization.
“Our current discussions include major US anesthesia product distributors, strategic partners and investors,” said Omeq Medical Chairman Carl Rickenbaugh, who has 30-plus years of executive experience in the medical-device space with companies such as CR Bard and Abbott Laboratories.
“We are now doing premarket activities ahead of a limited US launch early next year,” Margalit tells ISRAEL21c.
And even though the device already has FDA clearance, Margalit plans to provide further clinical data by conducting a large multicenter study in the United States to show how the EpiFinder device improves performance and successful placement.
Because the same technology could be used to assist in the accurate delivery of any medical instrument to any specific anatomical region, Margalit adds, Omeq will be exploring other clinical indications for its future pipeline. The first will likely be orthopedic injections.
For more information, click here.