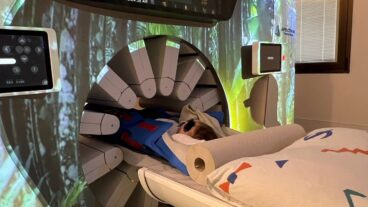
MTRE’s Allon Wrap has been clinically proven, and – according to the company – has been shown to significantly reduce morbidity, immunosuppression, coagulopathy, and shivering. At Schneider Children’s Medical Center in Petah Tikva, surgeons will not begin a 10-hour liver operation until the patient has been wrapped in a ThermoWrap.
The disposable wrap, which was designed by Israeli company MTRE Advanced Technologies, helps stabilize and control the temperature of a patient through surgery. It ensures that, despite the cool temperature of the operating room, and the introduction of anaesthesia into the body, the patient remains at a normally warm temperature throughout the procedure.
Staying warm is extremely important. Hypothermia is a serious problem after any long operation, and is directly related to immunosuppression, wound infection, problems with coagulation of the blood, and even problems with the heart. It also causes violent shivering, which is uncomfortable for a recovering patient.
Maintaining body temperature is especially problematic in patients undergoing cardiovascular surgery who must have their whole chest area, groin, and legs exposed. Children and babies are also seriously at risk. The problem is so severe in babies in fact, that if a baby’s temperature falls below a certain point during surgery, the surgeon will stop the procedure, and send the baby to the ICU to warm up, losing a valuable one or two hours in the process.
MTRE’s technology, called the Allon system after the founder’s son, is a one-piece garment that looks a bit like a large bandage, filled with water. It is modular in design so that it can be adapted for every type of operation. It can either be closed over a patient’s body with preattached adhesive strips – covering up to 86 percent of the surface of the skin, or, where large areas of the body need to be exposed for surgery, can be glued to the skin with a special adhesive. Infants, who have a large head area compared to the rest of the body, have a special cap that fits onto a tailor-made suit.
The wrap, which can be used for heating or cooling between 30-40 degrees centigrade, is then attached to a control unit with a microprocessor, which modulates the temperature of the water in the suit according to the patient’s temperature. The wrap has been clinically proven, and – according to the company – has been shown to significantly reduce morbidity, immunosuppression, coagulopathy, and shivering. At the same time, this helps reduce healthcare costs by reducing hospital stays, and post-surgery medications.
The idea for the wrap came from Dr. Igal Kushnir, a paediatrician, who originally set out to develop a new way to treat children suffering from high fever. He set up MTRE in 1998 with a number of colleagues, and after analysing the potential markets, realised that body warming during surgery was a larger potential source of sales. Altogether, the Or Akiva company has raised $27 million, in several fund-raising rounds. The last was completed in January 2001 and raised $17.5m., at a company value of $50m. Investors include CBI (Clal Biotechnology Industries), Neurone Venture Capital Fund, Challenge Fund – Etgar, Technorov Holdings, Koonras Technologies and a group of private investors. Development of the body wrap was completed in 2000, and later that year the company was granted FDA approval. Sales began in 2001. This year the company expects to see $1m. in revenues and next year the figure should reach $3-4m., with break even point expected in 2005.
While the original application was to warm patients, MTRE has recently entered a new field involving cooling the patient – induced hypothermia. The use of hypothermia is one of the most exciting areas in medicine today. While the idea has been around since the 1950s, it is only in the last two years that the treatment has really taken off. In July the powerful American Heart Association (AHA) endorsed the treatment to prevent brain damage in victims of cardiac arrest.
Those who can benefit from induced hypothermia include stroke victims, and patients who suffer cardiac arrest, although it can also be used as a technique in neurosurgery and for traumatic brain injury. Today in the US, some 500,000 people have strokes each year, while between 250,000 to 300,000 suffer a cardiac arrest. The survival rate of patients who suffer a cardiac arrest outside of hospital stands at just one to five percent. Among survivors, as with stroke victims, the frequency of some kind of severe neurological damage is high. Therapeutic hypothermia, however, has been shown to protect the neurological functions of the brain by slowing metabolism, and reducing the demand for oxygen. This slows the process of cell death.
The body wrap, which costs $80 to $100 per wrap, and $8,000 for the control unit, is as efficient at cooling as it is at heating, says Amir Shiner, MTRE’s chief technology officer. He says the company’s goal is to encourage hospitals to begin using the wrap the moment the patient arrives at an emergency room, since the quicker the cooling begins, the less cells are damaged. With MTRE’s device, cooling takes between one to two and half hours. In the next stage the company hopes that the wrap can even be introduced into ambulances, so that patients can be treated the moment they are picked up.
“This is very important in places like Europe or America where you might be as long as two hours in an ambulance,” says Shiner.
Luckily for MTRE, the company does not have to educate the market. “The market is educating itself,” admits Shiner, who notes that at a recent conference, hypothermia was recognised as one of the most established new treatments.
“People are very responsive to the AHA recommendations,” he notes. “Nobody wants to be left behind.”
In terms of competition MTRE stands out from the crowd. In the heating market, the only existing alternatives are fairly primitive in nature. Augustine Medical, a US company, sells a convective air blanket which blows hot air on to a patient through tiny holes in the blanket.
“This is good for short, uncomplicated procedures, but not so good for heart patients because of the large areas of the body that cannot be covered,” says Shiner. Other methods include a heated gel mattress and overhead radiators.
In the induced hypothermia market, MTRE is up against a new breed of companies, many of which were founded, like MTRE, in 1998, or 1999. Colorado company, Medivance, which raised $16m. in June last year, has developing cooling pads through which cold water circulates. Three other companies, Alsius Corp., Radiant Medical, and Innercool Therapies, which are all based in California, have developed similar technologies that cool from the inside using a large catheter which is inserted into an artery – usually the groin. The catheter is then pumped with cold sterilized saline which passes through the catheter and reduces a patient’s temperature quickly. The cooling process normally takes an hour.
“This approach is invasive and has dangerous complications like infections and clots associated with it,” says Shiner. “Not every patient can be treated this way.”
Shiner says he has talked to some anaethesiologists who claim they would never insert such a large catheter into a sick patient. On the other hand, he adds, “A lot of money has been invested in these companies and they are not stupid. They know what they are doing.” Alsius, for example, is backed by Medtronic.
“Apart from us, no one in the market actually regulates a patient’s temperature. Our device is the first to do it and is still considered the best,” says Shiner.
While MTRE has a good product in an interesting market, things have not always been easy. At the start of this year, the company laid off 23 workers from a staff of 40, introduced other cost-cutting measures, and began to try to push sales. A new CEO was appointed in February to oversee the reorganisation.
“There was a typical mistake by the founders and management who created a large expectation on the sales side that didn’t materialize quickly enough,” admits Shiner. “Expectations were too high, and too optimistic.”
Part of the problem is that entering the medical market is never easy, particularly with such an innovative treatment as hypothermia. “Doctors see this is a superior product but when it comes to making a decision on purchasing a product that is more expensive than others they will think a lot about it,” says Shiner.
With the reorganization virtually complete, MTRE is now in the process of raising $5m. in a new round of fund-raising. So far, the company has raised $2m. out of this sum. Shiner says the company plans to use this money to focus on marketing its existing products, and developing new applications, including a personal cooling or heating system for the military. Shiner believes that 2004 will be the breakthrough year, especially for the cooling side of the business.
“We have a real opportunity here. The cooling market may not be as big as the OR (operating room) market, but the need is much more significant,” says Shiner.