Despite decades of malaria research, the disease still afflicts hundreds of millions and kills around half a million people each year – most of them children in tropical regions.
The best deterrent would be a vaccine composed of some of the parasite’s own proteins. However, those proteins identified as most promising for a malaria vaccine are unstable at tropical temperatures and require complicated, expensive cellular systems to produce them in large quantities.
Yet the vaccines are most needed in areas where refrigeration is lacking and funds to buy vaccines are scarce.
Ahead of World Malaria Day (April 25), a new approach developed at Israel’s Weizmann Institute of Science and tested at England’s University of Oxford shows promise as the basis of a future inexpensive malaria vaccine that can be stored at room temperature.
The RH5 protein — one of the malaria parasite’s proteins that has been tested for use as a vaccine — enables the parasite to anchor itself to the red blood cells it infects. Using the protein as a vaccine alerts the immune system to the threat without causing disease, allowing it to mount a rapid response when the disease strikes, and to disrupt the parasite’s cycle of infection.
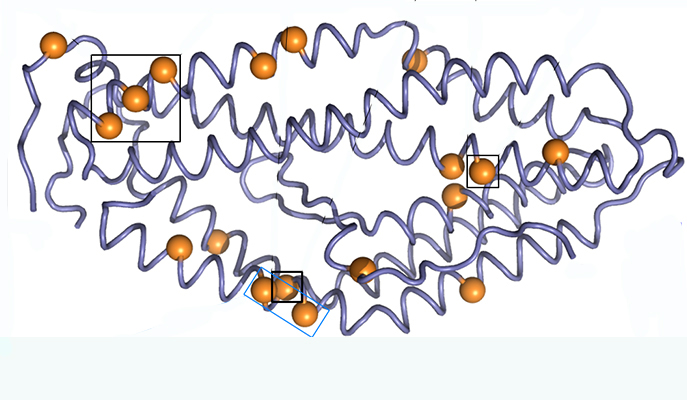
Weizmann research student Adi Goldenzweig and Dr. Sarel Fleishman of the Institute’s Biomolecular Sciences Department used computer-based protein design tools they have been developing in Fleishman’s lab to improve the usefulness of this protein by stabilizing it.
Goldenzweig invented a new way to “program” proteins used in vaccines against infectious diseases. Such proteins, because they are under constant attack by the immune system, tend to mutate from generation to generation. So the program she developed uses all the known information on different configurations of the protein sequence in different versions of the parasite.
“The parasite deceives the immune system by mutating its surface proteins. Paradoxically, the better the parasite is at evading the immune system, the more clues it leaves for us to use in designing a successful artificial protein,” she says.
Succeeds where others failed
The Israeli researchers sent the programmed artificial protein to a group in Oxford that specializes in developing a malaria vaccine.
This group, led by Prof. Matthew Higgins and Simon Draper, soon had good news: The results showed that, in contrast with the natural ones, the programmed protein can be produced in simple, inexpensive cell cultures, and in large quantities. This could significantly lower production costs.
In addition, the programmed protein is stable at temperatures of up to 50 degrees C, so it won’t need refrigeration. Best of all, in animal trials, the proteins provoked a protective immune response.
“The method Adi developed is really general,” says Fleishman. “It has succeeded where others have failed, and because it is so easy to use, it might be applied to emerging infectious diseases like Zika or Ebola, when quick action can stop an epidemic from developing.”
The study was published earlier this year in Proceedings of the National Academy of Science (PNAS).
Fleishman and his group are currently using their method to test a different strategy for treating malaria, based on targeting the RH5 protein itself and blocking its ability to mediate the contact between the parasite and human red blood cells.