An Israeli engineer has developed a new intubation device that may save billions of dollars in health expenses each year.

Like indoor plumbing, not much has changed over the last couple of decades with regard to tube feeding in the ICU. A simple pipe is inserted through the nose down into the stomach, and with the help of gravity, liquid nutrition and water from a bag are fed slowly into the tube. But shocking statistics from the US show that up to 15 percent of patients in ICUs will be infected with pneumonia brought on by reflux from feeding tubes.
Israeli engineer and entrepreneur Ofer Pintel tells ISRAEL21c that he believes he has the solution to fix this drastic problem, with the LunGuard feeding tube and monitor that he has devised. His device is poised to begin its first clinical trials.
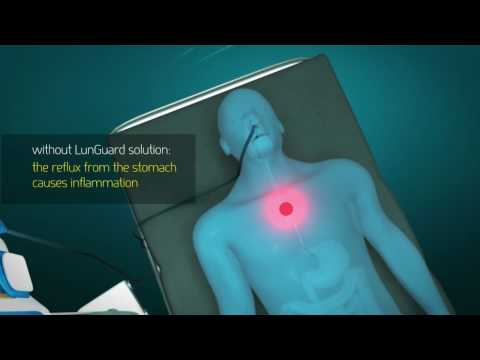
Reflux can cause a life-threatening situation when the stomach contents from a feeding tube shoot up the throat and get into the respiratory tract, causing infection. Expenses to the US healthcare system are enormous – some $10 billion dollars each year according to Pintel.
LunGuard’s first product is a peristaltic feeding tube that is capable of preventing reflux and is expected to significantly lower the occurrence of VAP (Ventilator Associated Pneumonia) in ventilated patients.
Saving billions in healthcare
In feeding tube technology, “nothing’s changed in the last 20 years,” says Pintel, LunGuard’s co-founder and CEO. Once a patient is intubated, the nursing staff has to manage suction and other mechanical methods to prevent reflux.
“A few nurses in the ICU have to do everything. They can’t manage. This is the main concern there; that these patients will get reflux and that it will lead to pneumonia.” Reflux is most dangerous in pre-term babies. At less than 32 weeks, infants are at serious mortal risk from any kind of infection.
A silicon tube, fitted with three balloon-like sphincters at its tip that receives the saliva going down, but in a carefully controlled manner prevents reflux from going back up, could be America’s solution to billions of dollars of healthcare expenses – a burden that usually rests on the hospitals. The tube is fitted to a high-tech monitor that controls the pressure within it.
Pintel, who has been involved in five medical start-ups in the past decade, says that HMOs reimburse expenses for the primary problem, but not those that are incurred to treat infections picked up at the hospital. These costs are shouldered by the hospitals themselves and it’s a big problem that they need to solve, he says.
To prevent reflux and infection, the feed rate is set at moderate, which is often slower than what a patient actually needs. And generally, no one monitors what’s happening during the four or five hours following feeding, Pintel explains. “Our solution can give information about peristaltic motion and the sphincter at the entrance of the stomach. We know that secretions crawl up to the respiration tract. Our solution prevents reflux continuously, and it’s safe. We are not just blocking the esophagus, but use a unique motion and balloons and a special feeding technology.”
Useful for emergencies, and long-term care
Moderate pressure control can prevent vomiting, so long as the instinct to vomit isn’t too strong. For now, sensors can be fine-tuned to achieve an optimal pressure in the feed tube, and in the future Pintel hopes to develop solutions with pH monitors and sensors that provide additional physiological information about the patient.
“It’s a solution that works for people with ALS [Lou Gehrig’s disease] and other degenerative muscular diseases,” he remarks, so that it can also help those suffering from muscular sclerosis and paralysis, and not just ICU patients. “In emergency situations, doctors are afraid of operating on a full stomach due to the reflux that could happen,” he notes, adding that “our solution can prevent reflux on a full stomach of food in an emergency situation.”
Based in Omer, in southern Israel, near Beersheba, LunGuard was established in 2009 with a grant from the Chief Scientist’s Office. Now included in the incubator Maayan Ventures, both the incubator and a VC firm in the UK have invested a total of $600,000 in the company. Pintel is the only full-time staff member on the payroll and he employs physicians and others as consultants to help with product development.
A prototype of the medical device is ready for the clinical studies on humans that are slated to be held in either Prague or Israel, as soon as the Health Ministry approves the request. If all goes according to plan, Pintel expects to receive both the CE and FDA stamps in 18 months to two years.
The company is currently seeking $2 million in financing to further the trials and to develop the prototype into a monitor-tube device which will probably be leased to hospitals, with disposable feed tubes costing around $25 per piece.