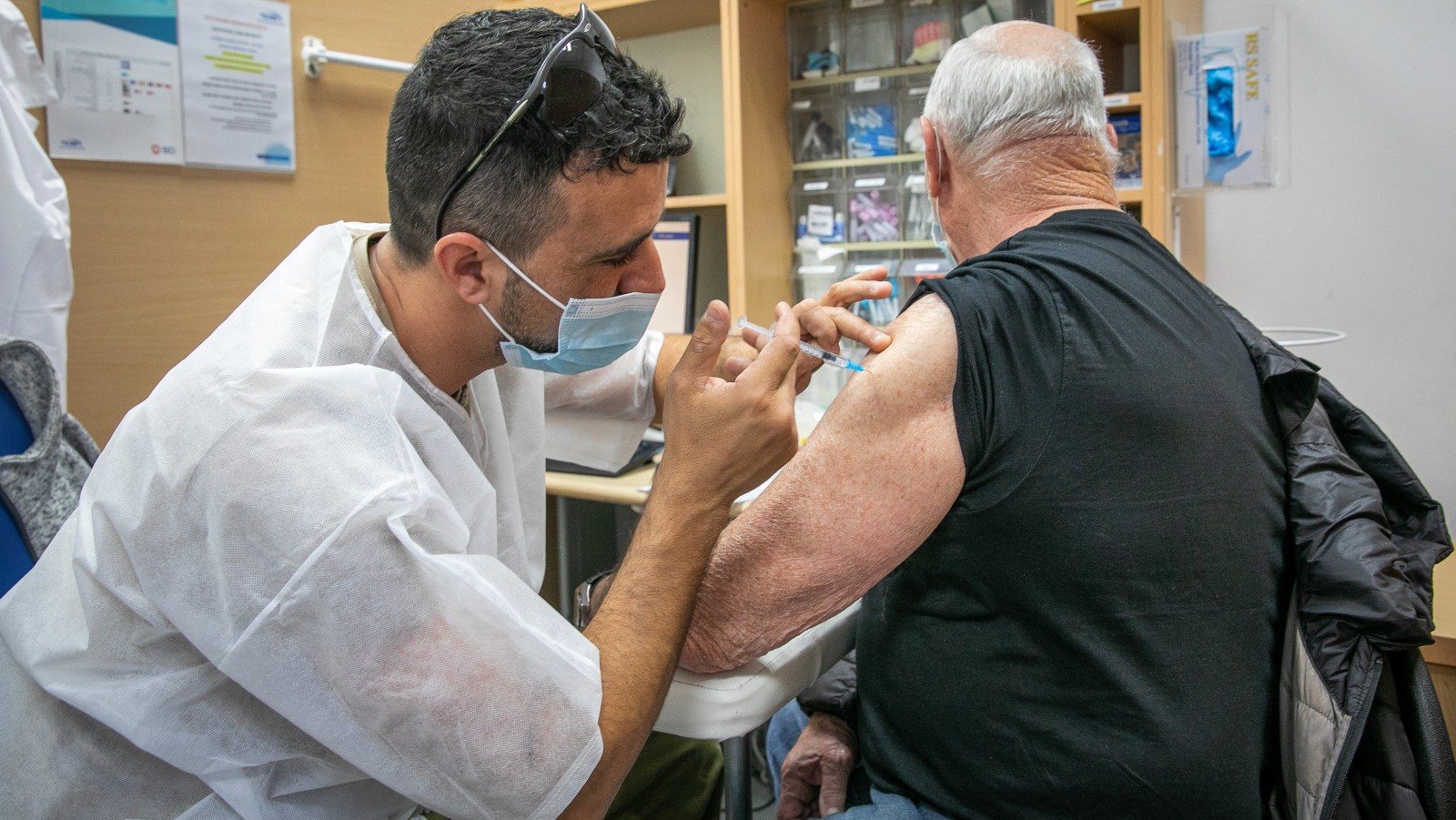
Israel’s Sheba Medical Center will be the first clinical trial site outside of the United States to study Covid-19 vaccine candidates formulated to protect against the Omicron variant of the SARS-CoV-2 coronavirus and other variants of concern.
Sponsored by Pfizer, the study will evaluate the safety, tolerability and immunogenicity of a higher dose of the Pfizer-BioNTech Covid-19 vaccine, the investigational Omicron-based vaccine and a combination of both vaccines given as a fourth dose in adults 60 years of age and older.
Approximately 200 participants will enroll in the study. Participants all previously received three doses of the current vaccine, with the most recent dose at least four months prior to enrollment.
These participants will be part of the larger, ongoing serological research at Sheba involving 6,000 medical personnel since the start of the worldwide vaccine campaign.
“This trial is extremely important as the world continues to deal with and battle these variants and perhaps others in the future. Vaccinations are the only way to roll back the global coronavirus pandemic,” said Dr. Gili Regev-Yochay, director of the Infection Prevention & Control Unit at Sheba Medical Center.
Yochay has been leading a separate trial studying the effects of a fourth dose of the original Pfizer vaccine.
Fighting for Israel's truth
We cover what makes life in Israel so special — it's people. A non-profit organization, ISRAEL21c's team of journalists are committed to telling stories that humanize Israelis and show their positive impact on our world. You can bring these stories to life by making a donation of $6/month.