If your knees feel fine, you have no reason to think about the meniscus.
Two of these cartilage pads cushion each knee in the area where the thighbone, shinbone and kneecap meet.
But if a meniscus gets damaged due to injury or illness — one of the most common types of knee injuries — you probably won’t be able to think of much else because of the pain.
Some patients can get by with pain management and physical therapy. Many others have the damaged meniscus removed, unfortunately increasing their risk of developing osteoarthritis.
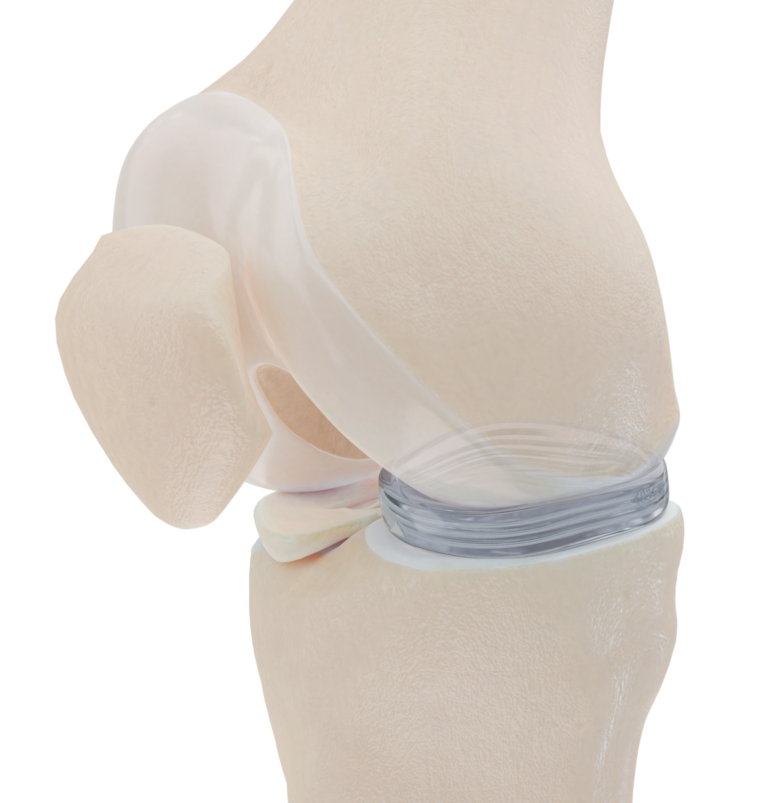
In 2019, ISRAEL21c reported on the world’s first artificial meniscus, NUsurface, made by Netanya-based Active Implants.
Since then, NUsurface has been implanted in close to 1,000 patients, mostly in the context of controlled studies, and is available commercially in Israel and many European countries. Pending FDA approval, the implant could launch in the US market in early 2024.
The first trial in-human implant of the product was done in 2008 on a 58-year-old Italian man and is still going strong, says Active Implants President Eran Ganz.
Now, the company is developing the next-gen NuSurface, intended to bring meniscus implants to an unprecedented level of performance.
Custom sizing and drug delivery
“We took this device we’re already selling and added patient-specific and drug-delivery features,” Ganz tells ISRAEL21c.
“Patient specific” means it’s not one-shape-fits-all like the current product.
“This is important because we’ve discovered the differing anatomy of patients is really affecting the performance of the device,” says Ganz.

The ability to control-release medication is important too.
“Most of the patients getting our implant have osteoarthritis and have cartilage degeneration,” Ganz explains. “We can restore the biomechanics, replacing the damaged or insufficient meniscus with our implant, but we are not doing anything active after that in terms of drug therapy.”
With the next iteration of NUsurface, “We can deliver one drug to help with the short-term healing and then a second drug that helps in the first eight months to delay development of cartilage degeneration.”
Active Implants has been working with a consortium of European clinicians, researchers and industry partners on the design for three years, with funding from the European Union’s Horizon 2020 program.
Enormous unmet need
Four medical experts from the consortium – from Belgium, Italy, Spain and Switzerland — came to Israel in November to help Active Implants strategize the path toward commercialization of the next-generation NUsurface.
“There is an enormous unmet need in the management of post-meniscectomy knee osteoarthritis in terms of treatment and especially prevention,” said Dr. Elizaveta Konfrom Italy, president of the International Cartilage Regeneration and Joint Preservation Society.
“The project demonstrates a huge ambition to address those needs, by developing novel technologies to treat young patients, as well as older patients who already developed post-meniscectomy osteoarthritis,” she said.
Ganz explains what Kon means by “young patients.”
“There is no other synthetic meniscus that can be used in patients aged 40 to 60, who are too old for biological regenerative surgical treatment but too young for joint replacement,” he says.
“This is a treatment gap we are seeking to fill with our solution, which can delay the need for joint replacement.”
The big need that Kon identified underlies the reason why Horizon 2020 granted 6 million euros to the consortium.

“We’ve done market analyses,” says Ganz, “and in the US alone the potential market is a million patients per year, about $6 billion.”
Long road ahead
Based on the results of animal studies in Israel, Active Implants is looking forward to the first in-human trials of the enhanced NUsurface implant.
Ganz estimates that it could take about five years until the device hits the market.
“Since it’s a drug-delivery device along with an implant, it’s difficult from a regulatory point of view and I expect some challenges,” he acknowledges.
Meanwhile, the first-gen NUsurface still has no direct competition, Ganz says.
The implant, manufactured in northern Israel, is made of medical-grade polycarbonate urethane (PCU) and mimics the natural structure of the meniscus with reinforcement fibers inside.
In addition to its headquarters in Netanya, Active Implants maintains offices in The Netherlands and in Memphis, Tennessee. The company has an American CEO, Henry Klyce, and global finance and board members.
“Much work is still ahead of us,” says Ganz. “But there is no doubt that we are at an important milestone in our journey to constantly improve the current technology, in order to continue helping many patients around the world.”
For more information, click here.