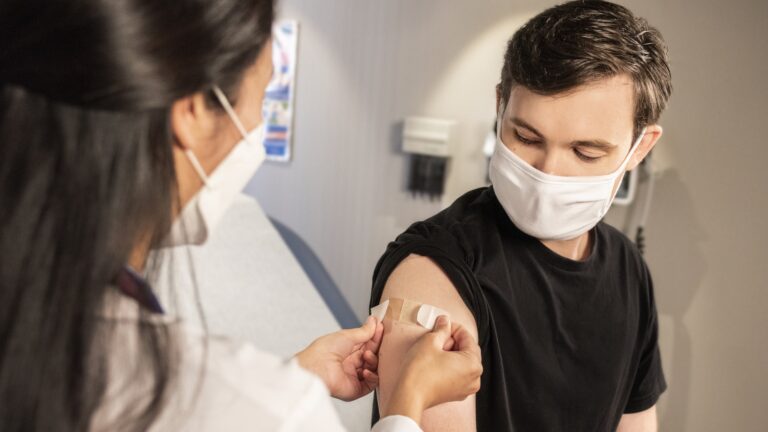
Today, Sheba Medical Center began the world’s first trial of a fourth dose of the Pfizer-BioNTech vaccine against Covid-19.
Sheba, the largest medical center in Israel and the Middle East, recruited 150 employees for the study. Each had received a third “booster” dose in August but now has a low antibody count of below 700, according to a hospital spokesman.
Led by Dr. Gili Regev-Yochay, director of Sheba’s Infectious Disease Epidemiology Unit, the study was approved by the Health Ministry’s Helsinki Committee overseeing human medical trials.
Although the US Food and Drug Administration has not yet approved a fourth Pfizer shot, Israel’s Pandemic Expert Committee wants to administer this booster to medical personnel and citizens over age 60 because protection seems to wear off by six months.
Regev Yochay said her goal is to determine whether the fourth dose is effective in stimulating the production of antibodies and whether it is safety tolerated.
“This study is expected to shed light on the additional benefit of giving a fourth dose. We will [seek to] understand whether, and to whom, it is worth giving a fourth dose,” she said. “We will have safety data within a few days.”
The results will help guide Israel’s policy and that of the rest of the world as the Omicron variant is causing a new wave of infections. Many experts believe the current formulation of the vaccine is ineffective against Omicron.
The first to receive the shot was heart transplant surgeon Dr. Jacob Lavee, who said it was “one small jab in the shoulder but one giant leap for mankind in the global battle against the Covid infection.”
Israeli Prime Minister Naftali Bennett noted that “Israel is continuing to stand at the forefront of the global effort to deal with the pandemic. The citizens of Israel were the first in the world to receive the third dose of the Covid-19 vaccine and we are continuing to pioneer with the fourth dose as well.”