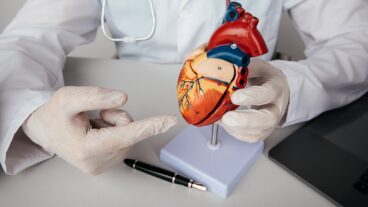
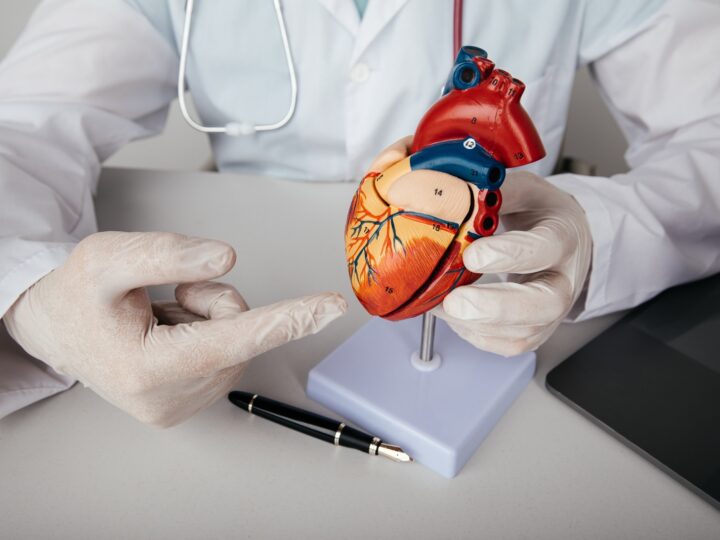
The year 2016 brought three major milestones for Israeli biomed company CartiHeal and its Agili-C cartilage and bone regeneration device: a $15 million investment led by Johnson & Johnson Innovation; European Union CE approval of Agili-C for cartilage regeneration; and now US Food and Drug Administration (FDA) approval of its Investigational Device Exemption (IDE) application.
The FDA approval clears the way for the Kfar Saba-based CartiHeal to begin a two-year pivotal study involving at least 250 patients, aimed to show Agili-C’s superiority over the current surgical standard of care (microfracture and debridement) in the treatment of cartilage or osteochondral defects in osteoarthritic knees and in knees without degenerative changes.
This multicenter, open-label, randomized and controlled trial is the first approved study of such broad indications using a single implant.
If the study is successful, the product could be on the American market in a few years, estimates CartiHeal founder and CEO Nir Altschuler.
“Over the last few years we’ve conducted a series of clinical studies in leading centers to learn which kind of patients can benefit from the Agili-C implant,” says Altschuler. “We believe that the Agili-C implant will prove to be an ideal treatment for a variety of cartilage lesions in patients who wish to return to a painless and active lifestyle, and currently don’t have good alternatives.”
Following its receipt of the CE Mark, Agili-C was implanted in more than 220 patients with cartilage lesions in the knee, ankle and/or great toe during a series of clinical trials conducted in Europe and Israel. Results demonstrated the potential for cartilage regeneration and remodeling of the underlying subchondral bone, along with pain and symptom relief.
“Pivotal studies performed to date were always focused on small, focal and isolated cartilage lesions in a narrowly defined patient group, which does not represent most of the ‘real-life’ cases,” explained Dr. Ken Zaslav, president of the International Cartilage Repair Society and a member of CartiHeal’s clinical advisory board.
“Based on the robust clinical data of the Agili-C implant, FDA has allowed, for the first time, treatment indications ranging from single focal defects to multiple defects in osteoarthritis of the knee, which is what we surgeons see on a daily basis. This exciting study will compare two control modes in a single arm: microfracture for the treatment of focal lesions and debridement for patients with osteoarthritis.”
Agili-C, an aragonite-based biodegradable scaffold, has been shown to promote regeneration of hyaline cartilage and remodeling of its underlying subchondral bone in a series of preclinical studies, without the use of cells or growth factors.
“We believe that it will provide a good solution for a vast unmet need, especially for patients with osteoarthritis who do not respond to conservative treatment while their condition is not severe enough to justify full joint replacement,” says Altschuler.