Forget analgesics and narcotics; the new Israeli treatment for backaches stimulates nerves non-invasively and electrically.
Chronic pain is one of the biggest reasons for sick days in America, and back pain is the most debilitating kind, something that eight out of 10 of us will experience in our lifetimes. Analgesics might work temporarily, but heavy-duty painkillers can lead to addiction.
A new Israeli company, Nervomatrix, just got US Federal Drug Administration (FDA) approval to start marketing its medical device for the relief of lower back pain. In the United States, this is big business: Approximately $80 billion is spent every year on treating back pain, and lower back pain in particular is the biggest mobility problem for the under-45 crowd.
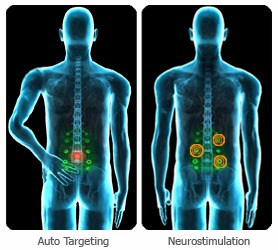
Using electrical signals emitted by the body, similar to the way EKGs can “read” heart health, the Nervomatrix device scans the back and can pinpoint where the pain is, expressed as low electrical resistance. It is at this point that the device applies a gentle electrical current to stimulate the nerves to reduce pain.
Device hitting US pain clinics
According to tests done by the company, the biggest jump in pain relief occurs between the first and second session, over a three-week, six-session treatment protocol. Now conducting additional clinical studies at New York University for pain practitioners to see for themselves, Nervomatrix expects to have its device in about 10 to 20 pain clinics in the United States over the next year.
Since initial outlay is high, and pain clinics don’t normally invest in expensive equipment, the device will be licensed to the clinics on a pay-per-use model. The average six-week treatment protocol could run in the $400 range depending on location, says the company’s CEO, Ori Kanner.
“I would assume that we’ll begin with conventional pain clinics,” he tells ISRAEL21c. “They tend to treat pain with conventional methods.”
Currently, there are no effective non-addictive painkillers on the market to treat chronic back pain, and Nervomatrix seems to be in a position to tap into a lucrative market.
Drug-free pain management
A treatment is considered to have been successful, if after six sessions, the pain doesn’t reoccur within a few days. The effects of Nervomatrix can last up to six months, and the company expects patients to come in for sessions no more than two or three times a year.
“We have to remember what we are treating,” says Kanner. “Our device treats the symptoms. If the reason for the symptoms isn’t found, the symptoms will re-occur. And more than 50 percent of lower back pain sufferers can find no reason for their pain. Most of the patients are in the group of sub-chronic, with one to three episodes of lower back pain a year.
The brain behind the invention is Dr. Miguel Gorenberg, a specialist in nuclear medicine. Kanner, who runs the business side of things, has a degree from the Technion-Israel Institute of Technology, and a master’s degree in radiation physics from the University of London.
Identifying chronic pain
The company’s device includes a back scanner and a nerve-ending locator. In future iterations, the device may be modified to use patient data to test if, in fact, there really is chronic pain. Currently there is no way for health practitioners to certify if chronic pain exists or not, and insurance companies are eager to discourage fraudulent claims.
While Nervomatrix has passed the necessary clinical trials to market the product in the United States, its first clinical trial was at the Bnai Zion Medical Center in Haifa, where 85 percent of the patients in the study reported an improvement in their condition.
Based in Netanya, Nervomatrix employs a staff of eight. The company’s product was in development since 2007 on a slim budget of $800,000, which includes grants from Israel’s Office of the Chief Scientist and from the incubator Targetech Innovation Center.