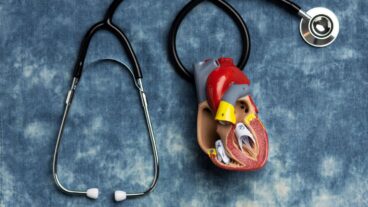
Active Implants’ TriboFit Buffer is a pliable, resilient cap that fits snuggly in the socket of the hipbone.Did you know that jogging puts five and a half times the body weight on your hip joints? And stumbling puts a peak load of eight times the body weight on the hips. Any high impact activity that repeatedly puts an extra load on the hip joints can eventually cause major damage and the need for hip surgery.
Today, the elderly are not the only patients. A growing percentage of patients are younger than ever before, and are paying the price for high impact sports. Both groups would like to know that their hip surgery will last and that they can return to an active life of jogging and basketball.
Now, Active Implants, an Israeli company has developed a new solution for hip reconstruction that mimics nature and promises to provide patients with just the long-term solution they are searching for.
Active Implants’ TriboFit Buffer is a pliable, resilient cap that fits snuggly in the socket in the hipbone that connects with the head of the femur to form the hip joint.
Made of a soft, pliable polycarbonate-urethane material invented only in the mid 1990s and already used successfully for cardiac surgery, it provides the same elasticity and cushioning as human cartilage. In addition it mimics the stress distribution functions of a healthy joint, and induces lubrication.
Eliminating wear
“A healthy hip joint comes with a shock absorber, a layer of cartilage and fluid, that helps to buffer the impact of significant loads and to establish normal stress distribution,” Stephen Bradshaw, president and CEO of Active Implants tells ISRAEL21c “With optimum lubrication, you can essentially eliminate wear.”
Active Implants was founded in 2004. The company’s technology is based on the research of Avraham Steinberg, an Israeli engineer, who studied at the University of Michigan. Steinberg first applied advanced polymers for the Israeli navy, to increase boat speed and payload. He began developing orthopedic devices in 1995, and is credited with 200 inventions in pliable implants.
Most current hip systems use polyethylene plastic that is 70 times more rigid than cartilage and discourages lubrication. Ceramic or metal-on-metal systems are cumbersome.
According to a study undertaken at Leeds University in England, the materials used by Active Implants in orthopedic devices showed little wear after five million cycles of testing.
Another study that took place at the University of Tennessee’s Health Science Center, found that the material used in the Tribofit buffer also reduces the risk that users will develop osteolysis, a bone-weakening condition that sometimes forces doctors to perform a second operation.
Osteolysis occurs when a toxin, a “bug,” enters the body like a Trojan horse and migrates to the joint. Studies show that infectious agents attach themselves to the polyethylene material, creating chemicals that destroy the bone around the hip joint and loosen the hip implants.
“Several renowned centers for orthopedic medicine in Europe have been involved in development and testing,” says Bradshaw. In particular, Professor Antonia Moroni and Dr. Sandro Gianinni of The Rizzoli Institute in Bologna, Italy and Professor Werner Siebert of the Kassel Clinic in Germany.
Extending implant life
“We believe we have developed a truly innovative hip system,” says Bradshaw, speaking from the company’s headquarters in Memphis, Tennessee, a hub of orthopedic innovations. “The TriboFit Buffer is a less invasive, bone conserving implant that is designed to improve function and extend implant life.”
Interest in the device is growing. An indication of the excitement it is generating is the decision by Jack Blair, a veteran expert in the field and one of the company’s first investors, to become the company’s chairman of the board in January this year. Blair recently served as chairman of the board of DJ Orthopedics, acquired by the Blackstone Group for $1.6 billion.
In its last round of financing, Active Implants successfully raised $20 million and is now preparing for another round. The company has already launched its TriboFit Buffer in Europe, the third largest market after the US and Japan, with more than 350,000 hip reconstruction procedures annually.
The device received the CE Mark in September 2006. The company hopes to gain FDA clearance in the US soon.
Meanwhile, Active Implants is moving ahead with a pliable structured artificial meniscus for the knee. It expects to introduce its implant solution early next year.