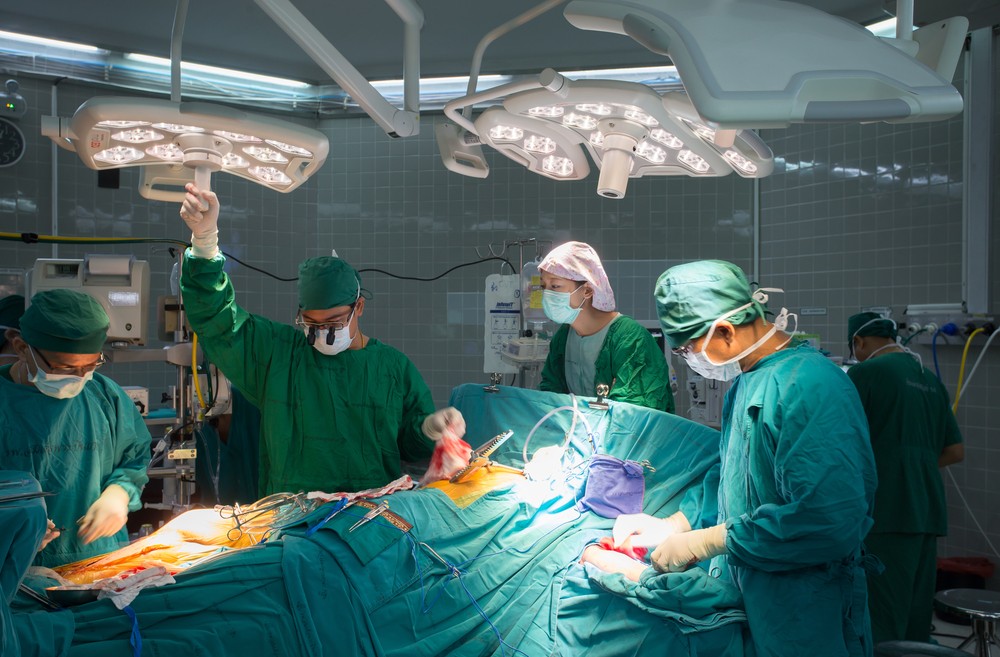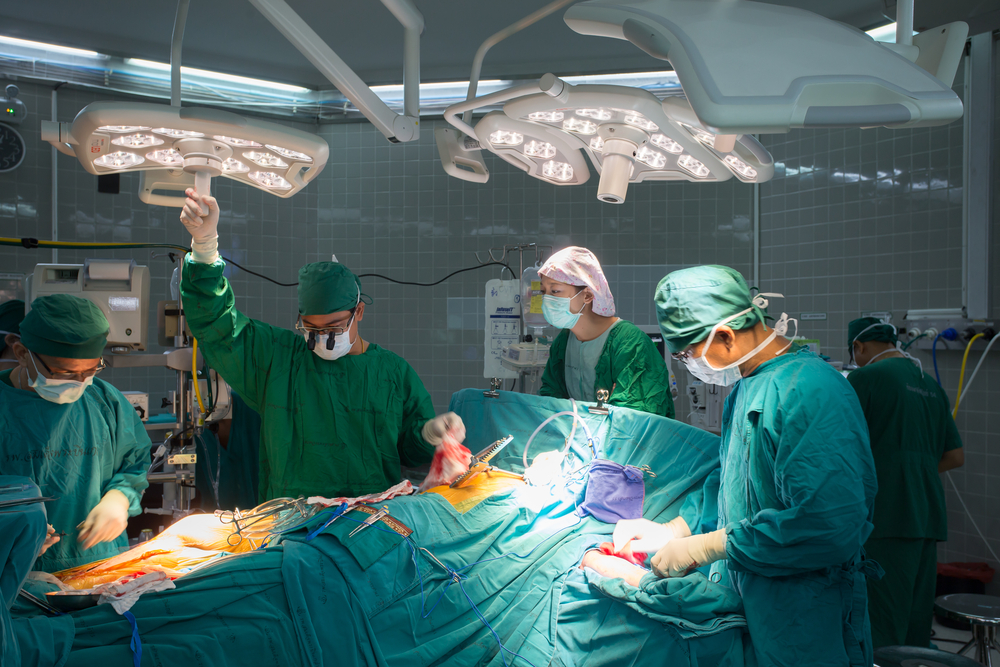
A mega acquisition deal worth roughly $929 million of Israeli replacement heart valve maker Valtech Cardio by HeartWare International has Israel’s startup sector buzzing with excitement.
The US-based heart technology company announced late yesterday that it has entered into a definitive agreement to acquire the privately held Or Yehuda company, which employs just 40 people, and specializes in the development of devices for mitral and tricuspid valve repair and replacement.
It is the biggest buy-out of an Israeli medical device company ever, and one of the biggest deals in any industry in Israel. It comes in the wake of a bumper year when in one week alone in January acquisitions of companies including Annapurna Labs, Red Bend Software, CloudOn and others reached $900 million.
“By joining HeartWare, we can more quickly and fully realize the potential of our pipeline technologies and further influence the underpenetrated markets that we serve,” said Amir Gross, founder and CEO of Valtech.
Terms of the deal will see Valtech shareholders receive an up-front consideration of 4.4 million shares of HeartWare common stock; 800,000 shares of HeartWare common stock, contingent upon CE Mark approval for Cardioband; and 700,000 shares of HeartWare common stock upon the earlier of first-in-man implants for either Cardioband tricuspid or CardioValve. The deal is said to be worth at least $929 million.
The buy-out is subject to regulatory approvals as well as HeartWare stockholder and Valtech shareholder approvals, the Massachusetts company said. The deal is expected to be closed later this year.
The mega-deal did not come out of nowhere. HeartWare and Valtech have been bedfellows since 2013 when the US company invested in the Israeli med-tech team.
Founded in 2005, Valtech Cardio has full, in-house development, manufacturing, and clinical research capabilities, and over 130 patents and patent applications. The company, which was founded by Gross and Yiftach Beinart in an incubator in Ariel, began life with a grant from the Office of the Chief scientist.
“We identified Valtech as having the broadest, most compelling portfolio several years ago, which led to an investment in 2013. This investment gave us a unique opportunity to observe Valtech’s significant progress across their portfolio of valve repair and replacement technologies. It is from this vantage point that we have concluded that Valtech’s platforms represent the most innovative and comprehensive portfolio of interventional and surgical products for mitral and tricuspid repair and replacement in development today,” said Doug Godshall, president and CEO of HeartWare.
Helping millions
Millions of people with heart disease are likely to benefit from this deal.
Valtech is a leader in the development of innovative surgical and transcatheter valve repair and replacement devices for the treatment of mitral valve regurgitation (MR) and tricuspid valve regurgitation (TR).
MR is a condition in which the mitral valve leaflets fail to close properly, allowing backflow of blood from the left ventricle into the left atrium during systole. Left untreated, severe MR can eventually lead to a significant deterioration in cardiac function and, eventually, death. In the US alone 4.2 million patients are affected by mitral valve disease, according to HeartWare.
Meanwhile, TR is estimated to affect 1.6 million patients in the US and complements the mitral patient population, as a significant percentage of patients suffer from both MR and TR.
HeartWare, which develops and manufactures miniaturized implantable heart pumps, or ventricular assist devices, to treat patients suffering from advanced heart failure, says this deal will provide it with a complementary portfolio of technologies to broaden the treatments it offers heart failure patients and enhance patient outcomes.
“Valtech provides HeartWare with commercial-stage products for mitral repair, as well as a robust technology pipeline, an advanced R&D center and an impressive, experienced team with a proven track record,” said Godshall.
Valtech’s portfolio includes Cardioband, the first interventional, transfemoral, direct annuloplasty system designed for mitral and tricuspid repair; Cardinal, a semi-rigid, adjustable annuloplasty ring system that provides surgeons with the ability to optimize annuloplasty results; CardioValve, a low-profile, transcatheter mitral valve replacement (TMVR) system with five degrees of steering maneuverability; and V-Chordal, a surgical and interventional chord replacement system for MR repair.
V-Chordal, which has been successfully evaluated in a first-in-man clinical trial, is expected to expand the range of percutaneous solutions offered for treating degenerative mitral valve pathologies and allow more patients to be treated without the risk of open-heart surgery.
“The mitral repair market is already a well-established and rapidly growing market with a significant, unmet, immediate clinical need. The Cardioband transfemoral annuloplasty system represents a more reproducible and predictable platform for mitral valve repair than existing solutions. We believe Cardioband will be a natural and clear selection as a first-line treatment for the broadest spectrum of MR patients, since it offers a safer option and, even in early clinical use, has already demonstrated a strong efficacy profile,” said Godshall.
“Valtech has benefited significantly from HeartWare’s early investment in our company. Since then, we have developed a strong relationship based on a shared mission to deliver transformative products to patients with advanced heart failure and degenerative heart conditions,” said Amir Gross, founder and CEO of Valtech.
“HeartWare’s existing market development experience and commercial infrastructure provide a compelling platform from which to launch multiple products worldwide, including a near-term launch of Cardioband in international markets following anticipated CE Mark approval this year. Together, we can offer clinical heart failure teams a compelling portfolio of surgical and interventional technologies to serve the advanced heart failure population.”