Up to one million people — mainly pregnant woman and young children — are killed each year by the Plasmodium falciparum parasite, which causes the most devastating form of human malaria.
Israeli researchers have now revealed the genetic trickery this deadly parasite deploys to escape attack by the human immune system.
The parasite replicates within the circulating blood of infected individuals and modifies the surface of the infected red blood cells. It hides from the immune system by selectively varying which surface protein, or antigen, it displays at any one time.
Hebrew University Prof. Ron Dzikowski has long believed that understanding exactly how the deadly Plasmodium falciparum parasite bypasses the immune system – and bypasses drug therapies as well — will open the door to a more effective battle plan against the malaria parasite, which infects about 250 million people worldwide.
“Others are looking for drugs or vaccines, but the parasite is always one step ahead of us,” he told ISRAEL21c in 2013, when he won a Bill & Melinda Gates Foundation grant for his team’s work in uncovering this genetic mechanism. “Our approach is to understand how these parasites evade immune attack, and then we can learn how to disrupt this ability.”
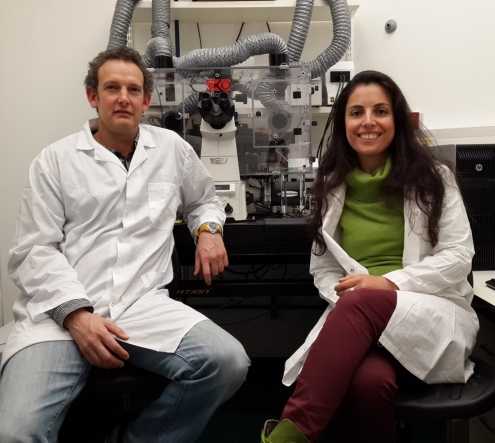
With his PhD student Inbar Amit-Avraham, Dzikowski recently found the key: At the precise moment in the cell cycle when a specific gene of the parasite is displayed, corresponding long noncoding RNA molecules (lncRNAs) incorporate themselves into DNA structures, determining how the parasite selects a single gene for expression while the rest of the family is kept silent.
In a series of genetic experiments with parasites, the researchers were able to activate silent genes by expressing their specific lncRNAs molecules, thus demonstrating clearly how they manage to pull off their microscopic game of hide and seek.
“We believe this breakthrough has exposed the tip of the iceberg in understanding how the deadliest malaria parasite regulates the selective expression of its genes, enabling it to evade the immune system,” Dzikowski said.
A fair playing field for the immune system
Dzikowski’s lab then collaborated with Eylon Yavin, at the Institute for Drug Research in the Hebrew University’s School of Pharmacy, to develop a novel way to interfere with lncRNAs.
Amazingly, they were able to suppress the actively displayed gene, and induce the parasite to switch on the expression of other genes.
“Understanding the mechanisms by which the parasite evades immunity takes us closer to finding ways to either block this ability, or to force the parasite to expose its entire antigenic repertoire and thus allow the human immune system to overcome the disease,” said Dzikowski.
The findings not only help pave the way for development of new therapies and vaccines for malaria, but from a broader perspective they also provide a new clue to scientists eager to break the code by which cells can express a single gene while keeping alternative genes silent.
The results of the research appear in the March 3, 2015 issue of Proceedings of the National Academy of Sciences (PNAS Early Edition).
The research was conducted at the Department of Microbiology and Molecular Genetics at the Institute for Medical Research Israel-Canada, in the Hebrew University’s Faculty of Medicine; and at the Sanford F. Kuvin Center for the Study of Infectious and Tropical Diseases at the Hebrew University-Hadassah Medical School.