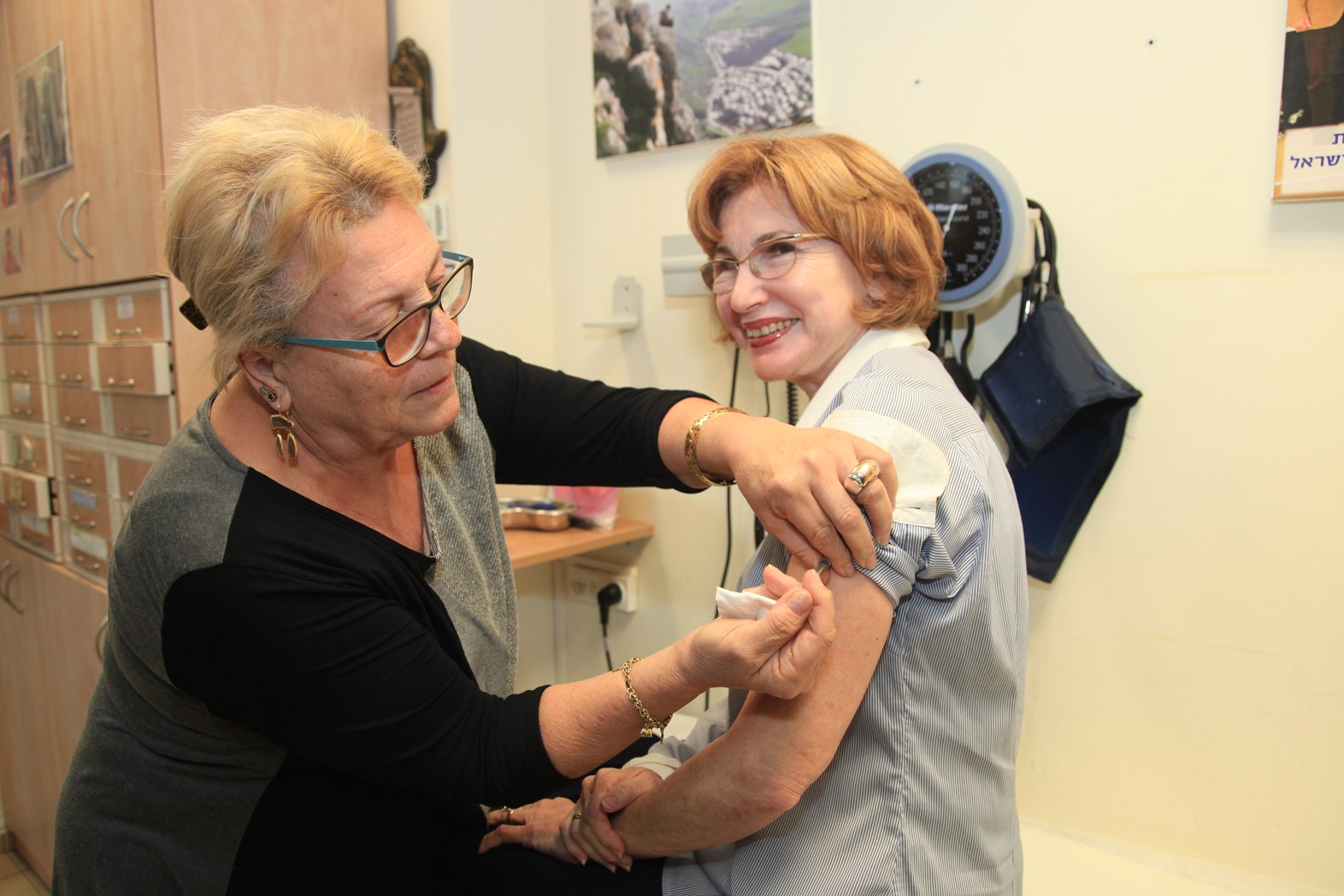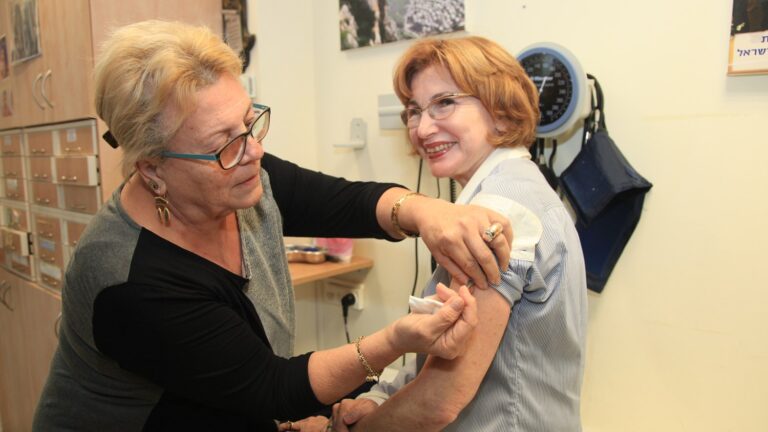
A phase 2 clinical trial of M-001, a universal influenza vaccine under development by BiondVax Pharmaceuticals in Ness Ziona, Israel, ended with positive preliminary results, the company announced last week.
M-001 was injected intramuscularly to volunteers between the ages of 50 and 65, followed three weeks later by the standard 2014-15 season trivalent influenza vaccine. M-001 was found to be effective, safe and well tolerated.
And as demonstrated in previous tests, recipients showed an immune response against strains not included in the standard flu shot, including the H3N2 strain that has caused this year’s epidemic in the United States. These results support BiondVax’s claim that M-001 provides broader and improved coverage against multiple influenza type A and B strains.
The company reports that the safety profile demonstrated for the 1-mg dose, higher than in previous trials, will green-light BiondVax’s upcoming clinical testing in Europe. The larger dose could be particularly beneficial to a population with weakened immunity, such as the elderly.
“We are very happy with these results, as they demonstrate the importance and value to society of developing M-001,” said BiondVax President and CEO Dr. Ron Babecoff. “This successful trial brings us one step further towards the phase 3 clinical trial.”
Fighting for Israel's truth
We cover what makes life in Israel so special — it's people. A non-profit organization, ISRAEL21c's team of journalists are committed to telling stories that humanize Israelis and show their positive impact on our world. You can bring these stories to life by making a donation of $6/month.