A simple filter could cut the high risk of stroke during a new minimally invasive operation to repair heart valves.
It’s a classic Catch-22: A revolutionary, minimally invasive heart repair procedure now being investigated in America, and already being performed by European doctors, could save the lives of older people who might not be able to withstand open-heart surgery to repair their heart valves. However, up to 15 percent of all patients undergoing this procedure — called TAVI, for transcatheter aortic valve implantation — suffer a stroke on the operating table.
Foreseeing the risk years ago as TAVI was being developed, Israeli cardiologist Dr. Dov Shimon invented a novel way to prevent stroke, which happens when hardened bits of calcium come dislodged during the TAVI procedure, passing through the heart and going into the brain.
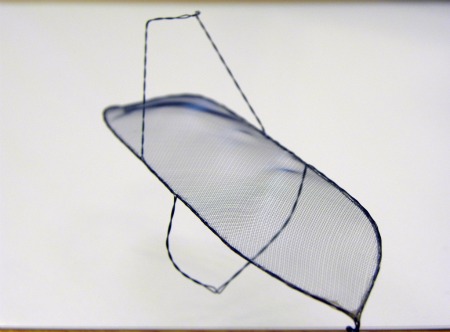
His innovation is a filter against these embolisms. Fitted onto the aortic arch, the medical device, now under development by the Israeli company SMT Research and Development in Herzliya Pituach, ensures that particles do not get into the blood flowing to the three main vessels leading from the heart.
The company of seven was founded in 2005 and has already raised $15 million, $10 million of which is from the healthcare equity fund OrbiMed. It is running its first human clinical trials in Europe, and similar clinical trials are to begin in the United States in 2012. In 15 surgeries performed in Holland using the SMT device, not one stroke has occurred.
A successful deflection system
Inserted before the operation and removed shortly after, the SMT filter appears to successfully prevent hardened artery materials from coming dislodged during TAVI, which holds great promise for many patients because it is performed through an artery in the leg.
“Studies of the last two years have shown significant incidence of stroke with this procedure,” SMT CEO Paul Zalesky tells ISRAEL21c. “These valves are so diseased that big chunks of calcium [break off] when they go in to do the procedure. Sometimes this material is knocked off, and breaks loose and goes into the brain.”
The SMT solution offers “a deflection system that sits on top of the arch of the aorta so any material will be deflected and not go to the brain,” he says.
Zalesky, who travels widely, and divides his time between Israel and California, recently presented the technology at the Paris Course on Revascularization, the second biggest conference in the world for cardiologists.
Physicians there told him they are eager to start using this device, he reports, but it must first get approval from the US Food and Drug Administration before it can be mass marketed.
Dr. Pieter Stella of the University Medical Center in Utrecht, the Netherlands, got a sneak preview by participating in the clinical trials.
“The SMT device is the first embolic protection device that I have seen that offers coverage of all three primary vessels feeding the brain. I have been pleased with our early results, and believe that the SMT deflector will help to ensure the continued market expansion for safe, effective TAVI procedures,” he says.