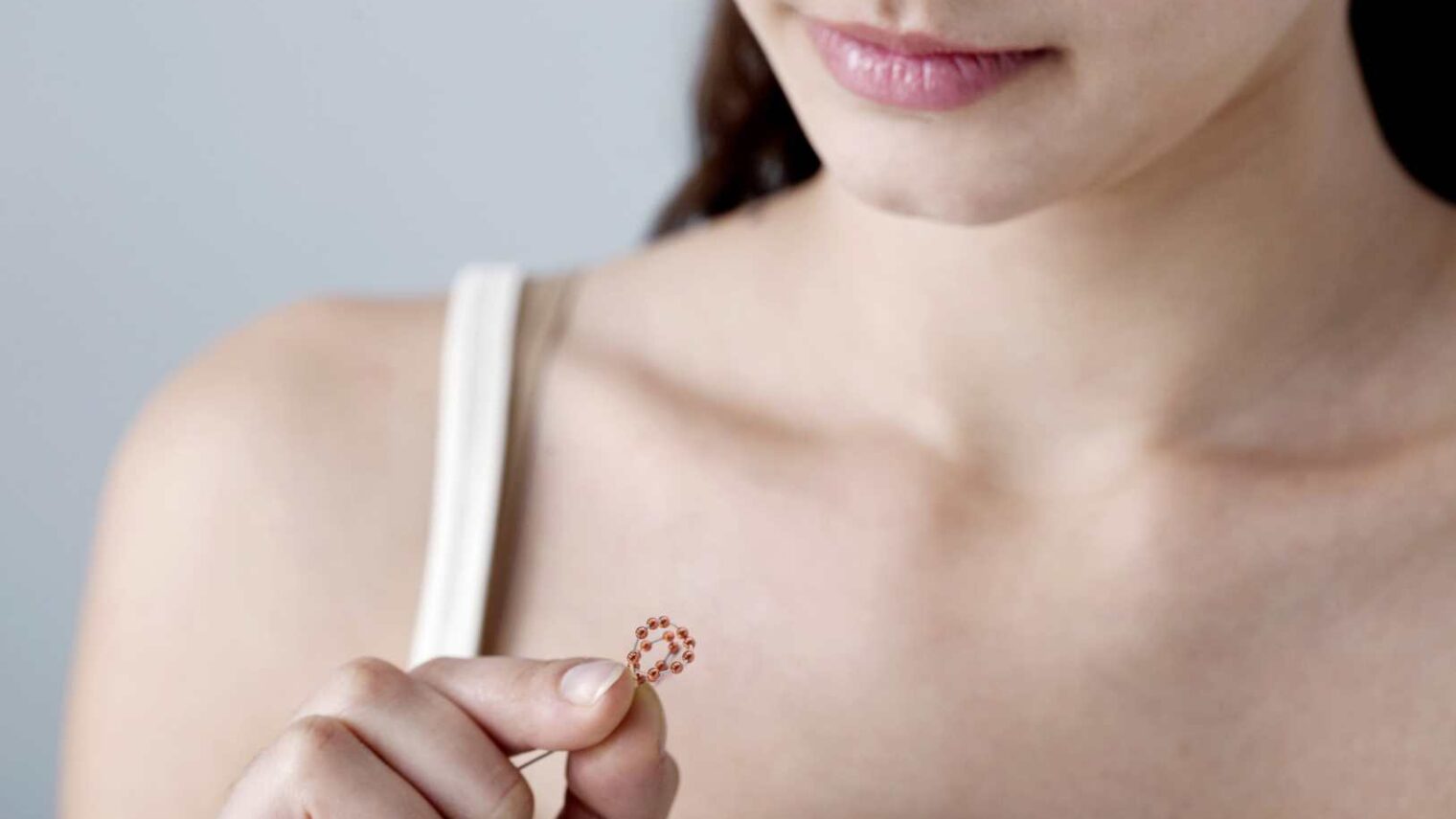
A contraceptive device from Israel called Ballerine has refined the intrauterine device (IUD) for greater comfort and safety, according to its developer.
Made by OCON Medical in Modi’in, Ballerine is the first product based on the trademarked IUB (Intra Uterine Ball) invented by senior gynecologist Dr. Ilan Baram, medical director of the company.
“The Ballerine is the first contraceptive device designed to address a quality-of-life issue before the issue of effectiveness,” Baram tells ISRAEL21c. “It’s common knowledge that inserting a copper device into the uterus will effectively prevent pregnancy but nobody seemed to take into consideration how women feel about it.”
The device, which had a proof of concept and safety study in Hungary, has been on the market since 2014, and is sold in Israel and 19 countries across Europe and Africa. It is spherical, soft and has no sharp edges, unlike the current rigid T-shaped devices.
“Until recently, people looked at the uterus as a flat triangle and a T-shaped IUD based on that notion would nicely fit. But the uterus really is a cavity that is viable and contracts constantly. Once you insert a T-shaped device it will probably end up mal-positioned and that is why 60 to 70 percent of women using an IUD have increased pain and bleeding,” Baram explains.
“This is the heart of the reason we needed a new kind of device.”
In the summer of 2008, Baram had a serendipitous idea that ball-shaped IUDs would greatly reduce the likelihood of mal-positioning. But how could such a shape pass through the very narrow cervical canal?
The solution was Nitinol, a metal alloy with unique elastic properties that is used in medical implants such as coronary stents. Nitinol can be “programmed” to retain a certain shape.
“When OCON was established in early 2011 we chose Nitinol for the IUB’s frame core and went on to design the gentlest shape to minimize risks and endometrial irritation,” says Baram, who together with CEO Ariel Weinstein gathered an international board of medical advisers for OCON.
“Since then we have developed additional Ballerine variants to fit different physiologies.”
The made-in-Israel device features several copper “pearls” strung on a flexible Nitinol wire frame. The frame is pre-loaded in a linear form inside the insertion tube; when it’s released in the uterus it coils into a three dimensional sphere.
“We were the first to globally introduce Nitinol to the uterus,” says Baram. “It’s a very sophisticated and elegant solution but at the same time very simple.”
The hormone-free Ballerine works on the same principle as other copper IUDs and is advertised as effective for up to five years before removal by a doctor, though future models could last up to 10 years.
Ongoing studies
Statistics on IUD use vary by country. IUDs are very popular in Asian and Scandinavian countries, while in Western Europe about one-third of women who use contraception choose an IUD, which is considered a long-lasting reversible contraceptive.
In the United States, IUDs fell out of favor due to a product that caused severe side effects in the 1970s – which turned out to be the fault of the attached removal strings rather than the device itself.
Baram says that in the last 10 years, American use of IUDs has grown from 1% to 14% of contraceptive users. So while the Ballerine has CE clearance for Europe, OCON is pursuing FDA clearance in the United States.
“We are going to start a large multi-center study mid-2018, under FDA guidelines, involving over a thousand women in the US and Europe. It will last several years with gradual publication of results. And expecting a positive outcome, we will then apply for FDA approval,” says Baram.
More than 50,000 units have been sold through distributors since late 2014 and fitted by gynecologists at an insertion-inclusive average patient cost of about €250. Ballerine has launched in Africa at a similar price; Baram hopes that partner organizations currently reviewing the product will be able to make Ballerine available to women who cannot afford this price.
Results of a study on women using Ballerine in Europe are soon to be published, but Baram says his own experience in Israel, where there have been over 1,000 insertions so far, has been very good. “Most women arrive for insertion by word of mouth,” he says.
“The interest that the company has raised in international drug companies is huge, which leads us to believe we are on the right path” he says.
OCON Medical is backed by Pontifax, RMI, Docor International, E. Burke Ross and Geddes Parsons. Its newest shareholder is Chemo Group, a global healthcare company whose Exeltis Women’s Health Business has been marketing Ballerine in several European countries since 2016.
The Chemo investment will accelerate OCON’s ability to make and market Ballerine as well as its R&D to develop other applications of the IUB platform.
“Two or three years ago we understood that the ball shape can be used for additional purposes by carrying other compounds, not only copper, into the uterus for treatment of certain conditions — perhaps even infertility,” Baram explains.
Leandro Sigman, chairman of the Chemo Group, has joined the board of the 22-employee company. “I am excited by OCON’s innovative IUB technology. It is being really well received by gynecologists and women seeking long-acting hormone-free contraception,” said Sigman.
For more information, click here.