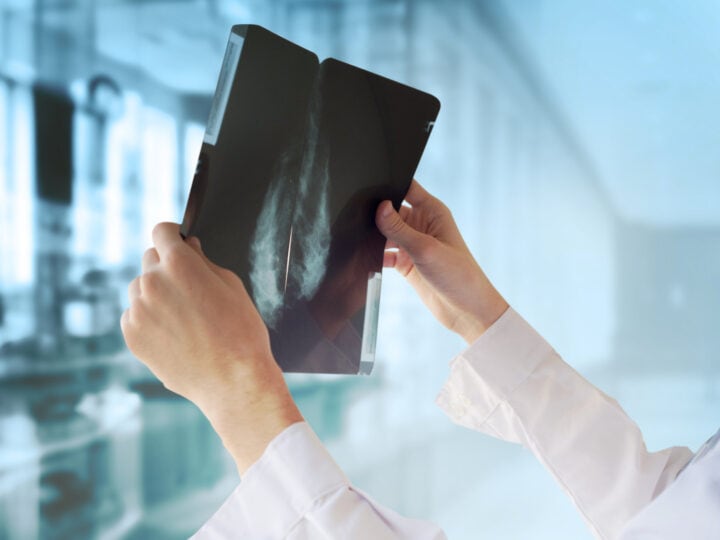
A groundbreaking point-of-care machine that determines whether an infection is viral or bacterial has received approval from the US Food and Drug Administration (FDA) for both children and adults.
Last year, MeMed received CE clearance for sales of its testing platform in Europe.
The MeMed BV test is an immune-based protein signature test that distinguishes, within 15 minutes, between a bacterial or viral infection from a blood sample.
This critical information enables physicians to know immediately if an antibiotic is called for, and to avoid antibiotic overuse.
“For those of us who care for acutely ill children, we have been waiting decades for accurate, rapid diagnostics to confidently guide the care of moderately ill children without a clear focus of infection or recognizable viral illness,” said Harvard Medical School Prof. Dr. Rich Bachur, chief of emergency medicine at Boston Children’s Hospital.
“This novel test offers promise to help differentiate those children with self-limited viral illness from those with possible bacterial infection, thereby supporting the judicious use of antibiotics,” Bachur said.
FDA clearance was based on a multi-center blinded clinical validation study enrolling over 1,000 children and adults and addresses goals laid out in the US National Action Plan for Combating Antibiotic Resistant Bacteria.
Headquartered in Haifa, MeMed has established its American base in Boston and is ramping up commercial activities across the United States.
Fighting for Israel's truth
We cover what makes life in Israel so special — it's people. A non-profit organization, ISRAEL21c's team of journalists are committed to telling stories that humanize Israelis and show their positive impact on our world. You can bring these stories to life by making a donation of $6/month.