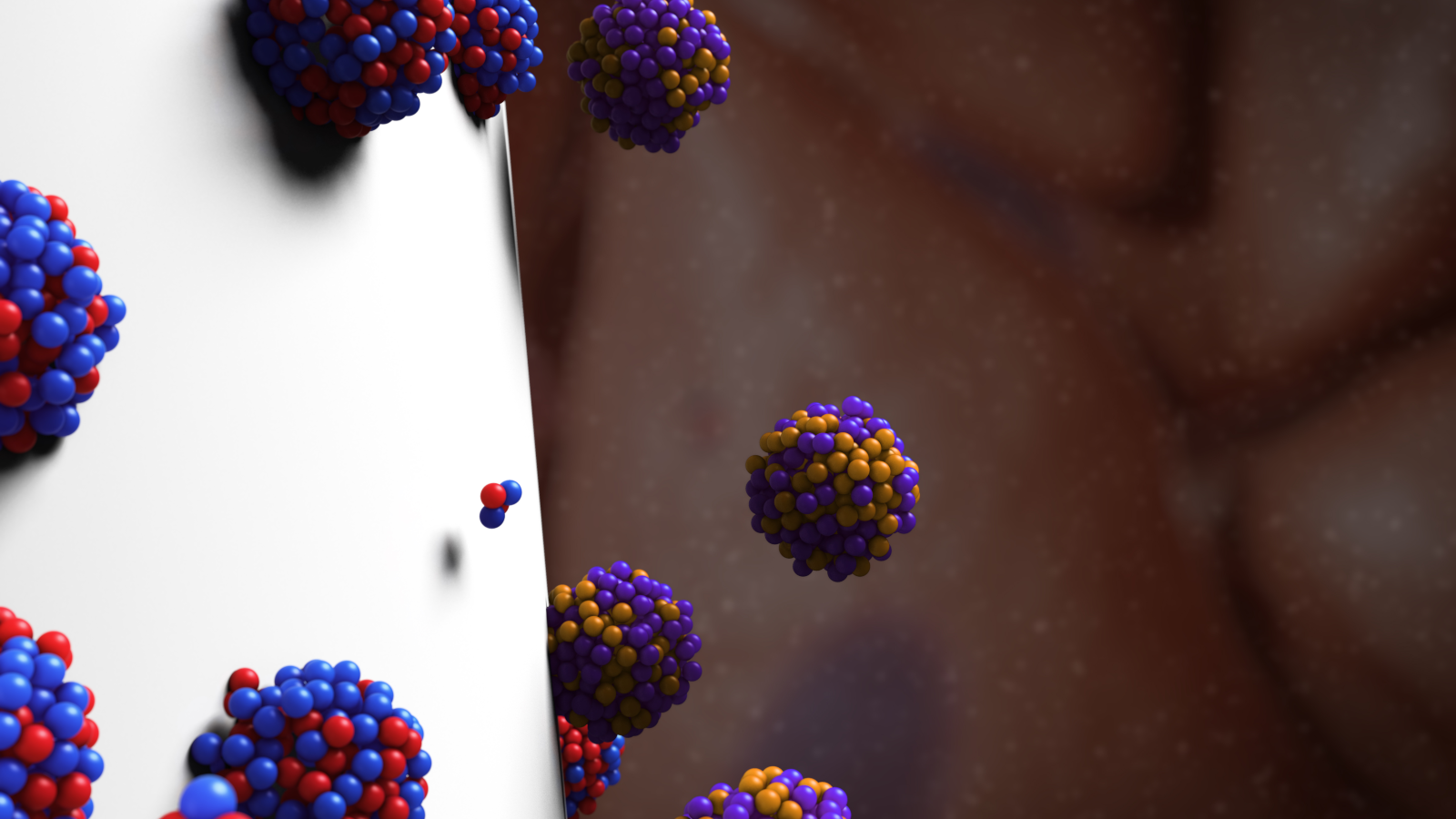
Results from the first clinical trial of Alpha Tau Medical’s revolutionary alpha radiation technology are in – and the news is encouraging for patients with aggressive, recurrent cancers.
Nearly 80 percent of 28 patients (78.6%) with squamous cell carcinoma received a complete response rate in managing their tumors; all patients responded to the treatment to some degree. They reported no systemic toxicity and only moderate local side effects that resolved within a few weeks.
The results are noteworthy given that more than 40% of the patients in the trial had already received radiation to the affected areas but had either not responded or had relapsed. In addition, prior forms of therapy had failed in 61% of the patients.
Alpha Tau is the only company to use alpha radiation to treat solid tumors. All other types of brachytherapy (where a sealed radiation source is placed inside or next to the treatment area) use beta or gamma radiation.
Alpha Tau’s technology uses less radiation and focuses it more efficiently on the tumor, preserving surrounding healthy tissue and causing fewer side effects. The approach was developed by Profs. Itzhak Kelson and Yona Keisari at Tel Aviv University. ISRAEL21c wrote about the company earlier this year.
This first human clinical trial, conducted in Israel and Italy, was designed to establish the safety, feasibility and efficacy of the company’s Alpha DaRT technology for patients with squamous cell carcinoma of the skin and head and neck region. Alpha DaRT “seeds” were placed on 31 lesions in 28 patients.
The results “confirm the promising findings from preclinical studies,” explained Rabin Medical Center radiation oncologist Aron Popovtzer, the principal investigator of the Israeli arm of the trial.
“The observed tumor response rates and survival metrics seem especially impressive given this elderly (median age 80.5 years) and heavily pretreated patient sample. Overall, these impressive outcomes serve as an excellent basis for future trials in other tumor types.”
The Italian arm of the trial was led by Dr. Salvatore Roberto Bellia from the IRST (Istituto Scientifico Romagnolo per lo Studio e la Curadei Tumori).
More trials are planned for additional types of skin cancer as well as pancreatic, prostate and breast cancer and will take place in Israel, Italy, France, Russia, UK, New York and Montreal.
“Once approved by the regulatory authorities, we can treat any kind of solid tumor,” Alpha Tau CEO Uzi Sofer told ISRAEL21c, “including cancers where there is no other chance and this is a patient’s last chance to live.”
The results were published in the International Journal of Radiation Oncology, Biology, and Physics, the official journal of the American Society for Radiation Oncology.