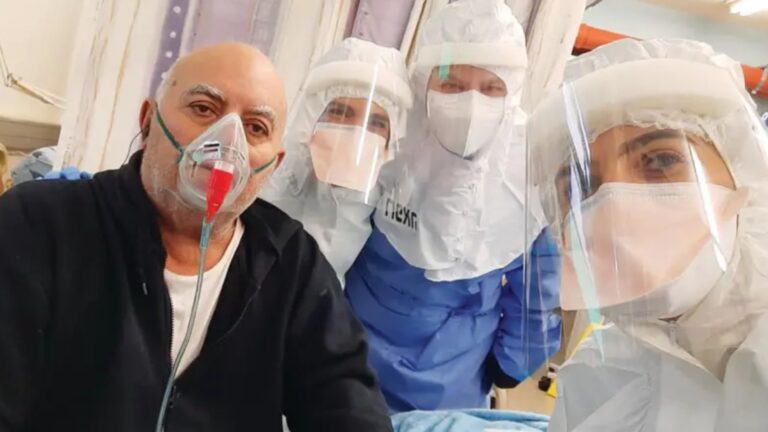
On October 1, the last of five Covid-19 intensive care patients who received the experimental drug Allocetra was discharged from Hadassah University Medical Center in Jerusalem.
This was the successful outcome of the world’s first clinical trial of Allocetra, made by clinical-stage immunotherapy company Enlivex Therapeutics of Ness Ziona, Israel, from engineered human cells donated by healthy individuals.
All five patients were discharged within about a week of receiving Allocetra and testing negative for the coronavirus. They reported no severe adverse events relating to the drug, and the therapy was well-tolerated.
Allocetra was developed based on the research of Enlivex chief scientific and medical officer Dr. Dror Mevorach, head of internal medicine and of one of Hadassah’s coronavirus wards.
On July 6, we included Enlivex as one of 13 promising Covid treatments emerging from Israel.
This novel immunotherapy medication treats organ dysfunction and acute multiple organ failure associated with sepsis and Covid-19, as well as solid tumors, by rebalancing the immune system following a cytokine storm.
“We have now treated 15 patients with Allocetra at our hospital, 10 with sepsis, and five with Covid-19,” said lead investigator Dr. Vernon van Heerden, chief of critical care at Hadassah.
“Based on the compelling preliminary results that demonstrated safety and an indication of efficacy of Allocetra in these complicated patients, Enlivex’s product candidate has the potential to benefit Covid-19 patients in severe or critical condition.”
Mevorach added: “We believe that the results of Allocetra treatment in these severe and critical Covid-19 patients represent a unique opportunity for Enlivex to contribute towards efforts aimed at combating the ongoing global Covid-19 pandemic.”
Earlier this year, Hadassah treated 12 Covid-19 patients with an experimental immunoglobulin derived from the antibody-rich plasma of recovered patients. The serum, prepared by Rehovot-based biopharmaceutical company Kamada, led to improvement in 11 of the 12 patients within 24 to 48 hours, and all 11 were discharged a few days later.
Kamada’s drug, however, is intended for moderately ill Covid-19 patients whereas Allocetra is for serious or critical cases. Enlivex believes that Allocetra, if approved, could potentially cover the gap that currently exists in treating severe or critical COVID-19 patients.
A larger Phase II clinical trial is planned, subject to regulatory approval.