Israeli scientists have discovered a new cellular pathway implicated in Gaucher disease — a genetic disorder most prevalent among the Ashkenazi Jewish population. The Weizmann Institute of Science researchers believe their findings, published recently in Nature Medicine, may lead to new treatments and management of this cruel disease.
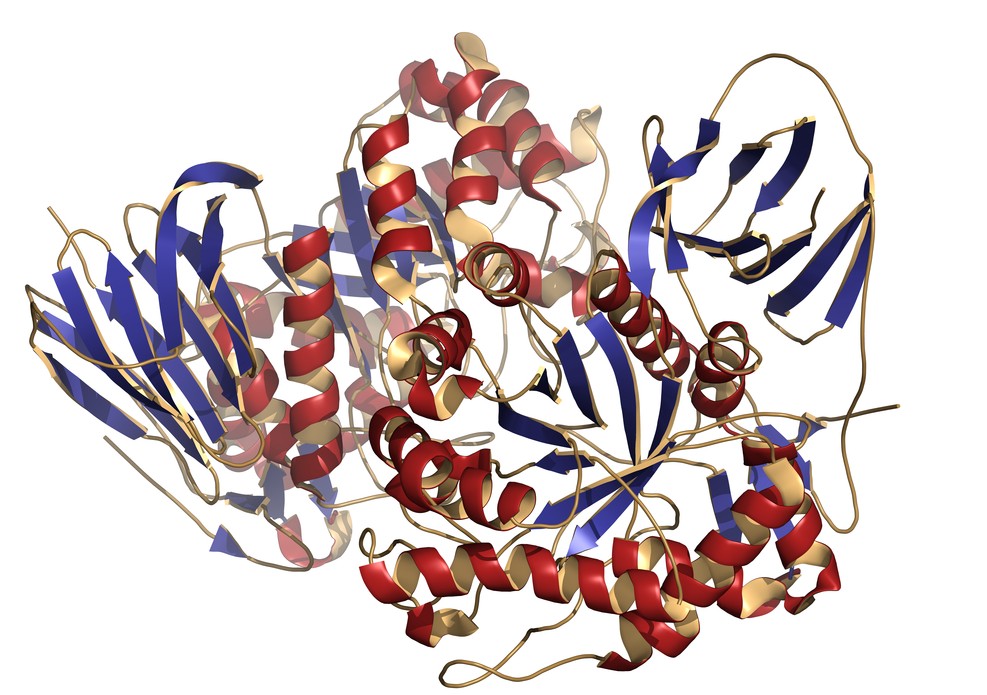
Gaucher disease is a genetic disorder caused by a defect in a particular enzyme needed to break down a fatty substance, or lipid, called glucocerebroside. This results in the accumulation of glucocerebroside in various cells and organs, which prevents them from working properly.
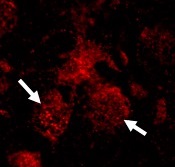
There are three subtypes of the disease: The most common form – Type 1 – is characterized by, among other symptoms, swelling and enlargement of the spleen and liver and disruption in the function of these organs, along with lung and bone problems. These symptoms can also affect individuals with Types 2 and 3 Gaucher disease, but what distinguishes them from Type 1 is the neurological involvement: Type 2 – the most severe form – causes extensive brain damage and death before two years of age, while Type 3 is a more progressive form of the disease that affects the brain, with patients often living into their early teens and adulthood.
But what exactly causes such a massive loss of nerve cells in Types 2 and 3 Gaucher disease?
It has recently come to light that a certain biochemical pathway, of which a protein called RIP3 is a key player, is involved in triggering the cell death and inflammatory processes that can have severe consequences in a number of diseases. Dr. Einat Vitner and M.Sc. student Ran Salomon, in the lab of Prof. Tony Futerman of the Biological Chemistry Department at the Weizmann Institute of Science, wondered whether this could also be one of the missing links in the understanding of the chain of molecular events leading to brain inflammation and nerve cell death in Gaucher disease.
To find out, they induced Gaucher disease in mice possessing the RIP3 protein, as well as in mice lacking RIP3. In mice lacking the RIP3 protein, they demonstrated not only a significant improvement in motor coordination and brain pathology but also improved liver and spleen function. Their lifespan was also remarkably increased from approximately 35 days to more than 170 days.
“These results are exciting, as they suggest a plausible new target for therapeutic intervention for all types of Gaucher disease; they have the potential, in the future, to greatly improve the patients’ quality of life,” says Vitner.
Although effective enzyme replacement therapy exists in which Gaucher patients are treated with injections of an intact version of the enzyme responsible for the normal breakdown of the lipid in healthy people, the cost of the lifelong treatment is approximately $200,000 per patient per year. Moreover, the enzyme is unable to get into the brain since it cannot cross the blood-brain barrier, rendering it ineffective in treating the neurological symptoms of Types 2 and 3 Gaucher disease.
“If successful, the new target could be used as either a complementary or alternative therapy for Gaucher disease, and with RIP3 proving to be a ‘hot’ cellular pathway in various pathologies, these results may also have implications in other neurodegenerative diseases, including related diseases such as Krabbe disease, and potentially other devastating brain diseases,” says Futerman.